Cellular repressor of E1A-stimulated genes attenuates cardiac hypertrophy and fibrosis
- PMID: 19413895
- PMCID: PMC4496144
- DOI: 10.1111/j.1582-4934.2008.00633.x
Cellular repressor of E1A-stimulated genes attenuates cardiac hypertrophy and fibrosis
Abstract
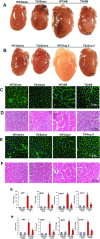
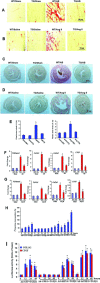
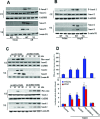
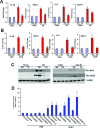
Cellular repressor of E1A-stimulated genes (CREG) is a secreted glycoprotein of 220 amino acids. It has been proposed that CREG acts as a ligand that enhances differentiation and/or reduces cell proliferation. CREG has been shown previously to attenuate cardiac hypertrophy in vitro. However, such a role has not been determined in vivo. In the present study, we tested the hypothesis that overexpression of CREG in the murine heart would protect against cardiac hypertrophy and fibrosis in vivo. The effects of constitutive human CREG expression on cardiac hypertrophy were investigated using both in vitro and in vivo models. Cardiac hypertrophy was produced by aortic banding and infusion of angiotensin II in CREG transgenic mice and control animals. The extent of cardiac hypertrophy was quantitated by two-dimensional and M-mode echocardiography as well as by molecular and pathological analyses of heart samples. Constitutive over-expression of human CREG in the murine heart attenuated the hypertrophic response, markedly reduced inflammation. Cardiac function was also preserved in hearts with increased CREG levels in response to hypertrophic stimuli. These beneficial effects were associated with attenuation of the mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase 1 (MEK-ERK1)/2-dependent signalling cascade. In addition, CREG expression blocked fibrosis and collagen synthesis through blocking MEK-ERK1/2-dependent Smad 2/3 activation in vitro and in vivo. Therefore, the expression of CREG improves cardiac functions and inhibits cardiac hypertrophy, inflammation and fibrosis through blocking MEK-ERK1/2-dependent signalling.
Figures


References
-
- Shindoh M, Higashino F, Kohgo T. E1AF, an etsoncogene family transcription factor. Cancer Lett. 2004;216:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

