Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase
- PMID: 19414066
- PMCID: PMC2777740
- DOI: 10.1016/j.freeradbiomed.2009.04.034
Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase
Abstract
Solar ultraviolet radiation (UVR) is the major etiological factor in skin carcinogenesis. However, in vivo studies demonstrate that mice exposed to arsenic and UVR exhibit significantly more tumors and oxidative DNA damage than animals treated with either agent alone. Interactions between arsenite and UVR in the production of reactive oxygen species (ROS) and stress-associated signaling may provide a basis for the enhanced carcinogenicity. In this study keratinocytes were pretreated with arsenite (3 microM) and then exposed to UVA (10 kJ/m(2)). We report that exposure to UVA after arsenite pretreatment enhanced ROS production, p38 MAP kinase activation, and induction of a redox-sensitive gene product, heme oxygenase-1, compared to either stimulus alone. UVR exposure resulted in rapid and transient NADPH oxidase activation, whereas the response to arsenite was more pronounced and persistent. Inhibition of NADPH oxidase decreased ROS production in arsenite-treated cells but had little impact on UVA-exposed cells. Furthermore, arsenite-induced, but not UVA-induced, p38 activation and HO-1 expression were dependent upon NADPH oxidase activity. These findings indicate differences in the mechanisms of ROS production by arsenite and UVA that may provide an underlying basis for the observed enhancement of redox-related cellular responses upon combined UVA and arsenite exposure.
Figures

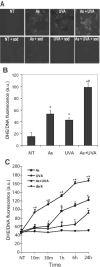



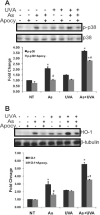
References
-
- Boukamp P. UV-induced skin cancer: similarities—variations. J Dtsch Dermatol Ges. 2005;3(7):493–503. - PubMed
-
- Nishigori C. Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci. 2006;5(2):208–214. - PubMed
-
- Ahmed NU, Ueda M, Nikaido O, Osawa T, Ichihashi M. High levels of 8-hydroxy-2'-deoxyguanosine appear in normal human epidermis after a single dose of ultraviolet radiation. Br J Dermatol. 1999;140(2):226–231. - PubMed
-
- Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6(3):561–570. - PubMed
-
- Baudouin C, Charveron M, Tarroux R, Gall Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002;18:341–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

