Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play
- PMID: 19414476
- PMCID: PMC2690506
- DOI: 10.1098/rstb.2009.0012
Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play
Abstract
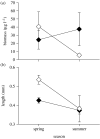
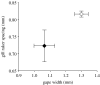
Interactions between natural selection and environmental change are well recognized and sit at the core of ecology and evolutionary biology. Reciprocal interactions between ecology and evolution, eco-evolutionary feedbacks, are less well studied, even though they may be critical for understanding the evolution of biological diversity, the structure of communities and the function of ecosystems. Eco-evolutionary feedbacks require that populations alter their environment (niche construction) and that those changes in the environment feed back to influence the subsequent evolution of the population. There is strong evidence that organisms influence their environment through predation, nutrient excretion and habitat modification, and that populations evolve in response to changes in their environment at time-scales congruent with ecological change (contemporary evolution). Here, we outline how the niche construction and contemporary evolution interact to alter the direction of evolution and the structure and function of communities and ecosystems. We then present five empirical systems that highlight important characteristics of eco-evolutionary feedbacks: rotifer-algae chemostats; alewife-zooplankton interactions in lakes; guppy life-history evolution and nutrient cycling in streams; avian seed predators and plants; and tree leaf chemistry and soil processes. The alewife-zooplankton system provides the most complete evidence for eco-evolutionary feedbacks, but other systems highlight the potential for eco-evolutionary feedbacks in a wide variety of natural systems.
Figures
References
-
- Abrams P.A. The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Syst. 2000;31:79–105. doi:10.1146/annurev.ecolsys.31.1.79 - DOI
-
- Arnott D.L., Vanni M.J. Nitrogen and phosphorus recycling by the zebra mussel (Dreissena polymorpha) in the western basin of Lake Erie. Can. J. Fish. Aquat. Sci. 1996;53:646–659. doi:10.1139/cjfas-53-3-646 - DOI
-
- Bailey J.K., Wooley S.C., Lindroth R.L., Whitham T.G. Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol. Lett. 2006;9:78–85. doi:10.1111/j.1461-0248.2005.00844.x - DOI - PubMed
-
- Bazely D.R., Jefferies R.L. Goose feces: a source of nitrogen for plant growth in a grazed salt marsh. J. Appl. Ecol. 1985;22:693–703. doi:10.2307/2403222 - DOI
-
- Benkman C.W. The selection mosaic and diversifying coevolution between crossbills and lodgepole pine. Am. Nat. 1999;153:S75–S91. doi:10.1086/303213 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

