Long-lived ames dwarf mice are resistant to chemical stressors
- PMID: 19414510
- PMCID: PMC2981464
- DOI: 10.1093/gerona/glp052
Long-lived ames dwarf mice are resistant to chemical stressors
Abstract
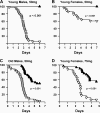
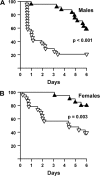
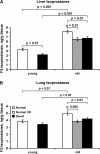
To probe the connection between longevity and stress resistance, we compared the sensitivity of Ames long-lived dwarf mice and control littermates with paraquat, diquat, and dobutamine. In young adult animals, 95% of male and 39% of female controls died after paraquat administration, but no dwarf animals died. When the experiment was repeated at an older age or a higher dosage of paraquat, dwarf mice still showed greater resistance. Dwarf mice also were more resistant to diquat; 80% of male and 60% of female controls died compared with 40% and 20% of dwarf mice, despite greater sensitivity of dwarf liver to diquat. Dwarf mice were also less sensitive to dobutamine-induced cardiac stress and had lower levels of liver and lung F(2)-isoprostanes. This is the first direct in vivo evidence that long-lived Ames dwarf mice have enhanced resistance to chemical insult, particularly oxidative stressors.
Figures
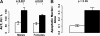
References
-
- Schaible R, Gowen JW. A new dwarf mouse. Genetics. 1961;46:896.
-
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. - PubMed
-
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58(4):291–296. - PubMed
-
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39(4):277–284. - PubMed
-
- Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72(5):653–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

