Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome
- PMID: 19414552
- PMCID: PMC2715042
- DOI: 10.1084/jem.20082242
Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome
Abstract
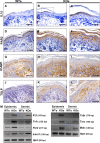
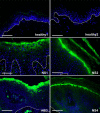
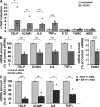
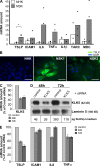
Netherton syndrome (NS) is a severe genetic skin disease with constant atopic manifestations that is caused by mutations in the serine protease inhibitor Kazal-type 5 (SPINK5) gene, which encodes the protease inhibitor lymphoepithelial Kazal-type-related inhibitor (LEKTI). Lack of LEKTI causes stratum corneum detachment secondary to epidermal proteases hyperactivity. This skin barrier defect favors allergen absorption and is generally regarded as the underlying cause for atopy in NS. We show for the first time that the pro-Th2 cytokine thymic stromal lymphopoietin (TSLP), the thymus and activation-regulated chemokine, and the macrophage-derived chemokine are overexpressed in LEKTI-deficient epidermis. This is part of an original biological cascade in which unregulated kallikrein (KLK) 5 directly activates proteinase-activated receptor 2 and induces nuclear factor kappaB-mediated overexpression of TSLP, intercellular adhesion molecule 1, tumor necrosis factor alpha, and IL8. This proinflammatory and proallergic pathway is independent of the primary epithelial failure and is activated under basal conditions in NS keratinocytes. This cell-autonomous process is already established in the epidermis of Spink5(-/-) embryos, and the resulting proinflammatory microenvironment leads to eosinophilic and mast cell infiltration in a skin graft model in nude mice. Collectively, these data establish that uncontrolled KLK5 activity in NS epidermis can trigger atopic dermatitis (AD)-like lesions, independently of the environment and the adaptive immune system. They illustrate the crucial role of protease signaling in skin inflammation and point to new therapeutic targets for NS as well as candidate genes for AD and atopy.
Figures



References
-
- Candi E., Schmidt R., Melino G. 2005. The cornified envelope: a model of cell death in the skin.Nat. Rev. Mol. Cell Biol. 6:328–340 - PubMed
-
- Swartzendruber D.C., Wertz P.W., Kitko D.J., Madison K.C., Downing D.T. 1989. Molecular models of the intercellular lipid lamellae in mammalian stratum corneum.J. Invest. Dermatol. 92:251–257 - PubMed
-
- Green K.J., Simpson C.L. 2007. Desmosomes: new perspectives on a classic.J. Invest. Dermatol. 127:2499–2515 - PubMed
-
- Egelrud T. 2000. Desquamation in the stratum corneum.Acta Derm. Venereol. Suppl. (Stockh.). 208:44–45 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

