In vivo pharmacodynamic characterization of a novel plectasin antibiotic, NZ2114, in a murine infection model
- PMID: 19414576
- PMCID: PMC2704636
- DOI: 10.1128/AAC.01584-08
In vivo pharmacodynamic characterization of a novel plectasin antibiotic, NZ2114, in a murine infection model
Abstract
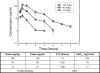
NZ2114 is a novel plectasin derivative with potent activity against gram-positive bacteria, including multiply drug-resistant strains. We used the neutropenic murine thigh infection model to characterize the time course of antimicrobial activity of NZ2114 and determine which pharmacokinetic/pharmacodynamic (PK/PD) index and magnitude best correlated with efficacy. Serum drug levels following administration of three fourfold-escalating single-dose levels of NZ2114 were measured by microbiologic assay. Single-dose time-kill studies following doses of 10, 40, and 160 mg/kg of body weight demonstrated concentration-dependent killing over the dose range (0.5 to 3.7 log(10) CFU/thigh) and prolonged postantibiotic effects (3 to 15 h) against both Staphylococcus aureus and Streptococcus pneumoniae. Mice had 10(6.3) to 10(6.8) CFU/thigh of strains of S. pneumoniae or S. aureus at the start of therapy when treated for 24 h with 0.625 to 160 mg/kg/day of NZ2114 fractionated for 4-, 6-, 12-, and 24-h dosing regimens. Nonlinear regression analysis was used to determine which PK/PD index best correlated with microbiologic efficacy. Efficacies of NZ2114 were similar among the dosing intervals (P = 0.99 to 1.0), and regression with the 24-h area under the concentration-time curve (AUC)/MIC index was strong (R(2), 0.90) for both S. aureus and S. pneumoniae. The maximum concentration of drug in serum/MIC index regression was also strong for S. pneumoniae (R(2), 0.96). Studies to identify the PD target for NZ2114 utilized eight S. pneumoniae and six S. aureus isolates and an every-6-h regimen of drug (0.156 to 160 mg/kg/day). Treatment against S. pneumoniae required approximately twofold-less drug for efficacy in relationship to the MIC than did treatment against S. aureus. The free drug 24-h AUCs/MICs necessary to produce a stasis effect were 12.3 +/- 6.7 and 28.5 +/- 11.1 for S. pneumoniae and S. aureus, respectively. The 24-h AUC/MIC associated with a 1-log killing endpoint was only 1.6-fold greater than that needed for stasis. Resistance to other antimicrobial classes did not impact the magnitude of the PD target required for efficacy. The PD target in this model should be considered in the design of clinical trials with this novel antibiotic.
Figures





References
-
- Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. - PubMed
-
- Ambrose, P. G., J. B. Anon, S. M. Bhavnani, O. O. Okusanya, R. N. Jones, M. R. Paglia, J. Kahn, and G. L. Drusano. 2008. Use of pharmacodynamic endpoints for the evaluation of levofloxacin for the treatment of acute maxillary sinusitis. Diagn. Microbiol. Infect. Dis. 61:13-20. - PubMed
-
- Andes, D., and W. A. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. - PubMed
-
- Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1-12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

