A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis
- PMID: 19414595
- PMCID: PMC2698746
- DOI: 10.1128/MCB.01164-08
A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis
Abstract
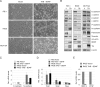
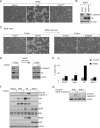
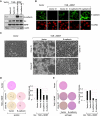
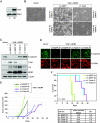
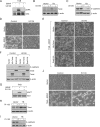
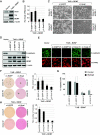
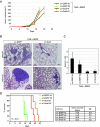
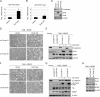
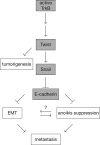
In a genomewide anoikis suppression screen for metastasis genes, we previously identified the neurotrophic receptor tyrosine kinase TrkB. In mouse xenografts, activated TrkB caused highly invasive and metastatic tumors. Here, we describe that TrkB also induces a strong morphological transformation, resembling epithelial-mesenchymal transition (EMT). This required TrkB kinase activity, a functional mitogen-activated protein kinase pathway, suppression of E-cadherin, and induction of Twist, a transcription factor contributing to EMT and metastasis. RNA interference (RNAi)-mediated Twist depletion blocked TrkB-induced EMT-like transformation, anoikis suppression, and growth of tumor xenografts. By searching for essential effectors of TrkB-Twist signaling, we found that Twist induces Snail, another EMT regulator associated with poor cancer prognosis. Snail depletion impaired EMT-like transformation and anoikis suppression induced by TrkB, but in contrast to Twist depletion, it failed to inhibit tumor growth. Instead, Snail RNAi specifically impaired the formation of lung metastases. Epistasis experiments suggested that Twist acts upstream from Snail. Our results demonstrate that TrkB signaling activates a Twist-Snail axis that is critically involved in EMT-like transformation, tumorigenesis, and metastasis. Moreover, our data shed more light on the epistatic relationship between Twist and Snail, two key transcriptional regulators of EMT and metastasis.
Figures
References
-
- Barrett, S. D., A. J. Bridges, D. T. Dudley, A. R. Saltiel, J. H. Fergus, C. M. Flamme, A. M. Delaney, M. Kaufman, S. LePage, W. R. Leopold, S. A. Przybranowski, J. Sebolt-Leopold, K. Van Becelaere, A. M. Doherty, R. M. Kennedy, D. Marston, W. A. Howard, Jr., Y. Smith, J. S. Warmus, and H. Tecle. 2008. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg. Med. Chem. Lett. 186501-6504. - PubMed
-
- Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 284-89. - PubMed
-
- Becker, K. F., E. Rosivatz, K. Blechschmidt, E. Kremmer, M. Sarbia, and H. Hofler. 2007. Analysis of the E-cadherin repressor Snail in primary human cancers. Cells Tissues Organs 185204-212. - PubMed
-
- Behrens, J., K. M. Weidner, U. H. Frixen, J. H. Schipper, M. Sachs, N. Arakaki, Y. Daikuhara, and W. Birchmeier. 1991. The role of E-cadherin and scatter factor in tumor invasion and cell motility. EXS 59109-126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
