The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis
- PMID: 19414596
- PMCID: PMC2704745
- DOI: 10.1128/MCB.01867-08
The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis
Abstract
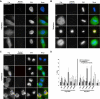
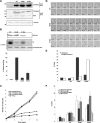
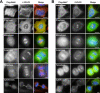
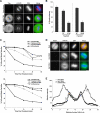
Nek6 and Nek7 are members of the NIMA-related serine/threonine kinase family. Previous work showed that they contribute to mitotic progression downstream of another NIMA-related kinase, Nek9, although the roles of these different kinases remain to be defined. Here, we carried out a comprehensive analysis of the regulation and function of Nek6 and Nek7 in human cells. By generating specific antibodies, we show that both Nek6 and Nek7 are activated in mitosis and that interfering with their activity by either depletion or expression of reduced-activity mutants leads to mitotic arrest and apoptosis. Interestingly, while completely inactive mutants and small interfering RNA-mediated depletion delay cells at metaphase with fragile mitotic spindles, hypomorphic mutants or RNA interference treatment combined with a spindle assembly checkpoint inhibitor delays cells at cytokinesis. Importantly, depletion of either Nek6 or Nek7 leads to defective mitotic progression, indicating that although highly similar, they are not redundant. Indeed, while both kinases localize to spindle poles, only Nek6 obviously localizes to spindle microtubules in metaphase and anaphase and to the midbody during cytokinesis. Together, these data lead us to propose that Nek6 and Nek7 play independent roles not only in robust mitotic spindle formation but also potentially in cytokinesis.
Figures






References
-
- Belham, C., J. Roig, J. A. Caldwell, Y. Aoyama, B. E. Kemp, M. J. Comb, and J. Avruch. 2003. A mitotic cascade of NIMA family kinases. J. Biol. Chem. 27834897-34909. - PubMed
-
- Capra, M., P. G. Nuciforo, S. Confalonieri, M. Quarto, M. Bianchi, M. Nebuloni, R. Boldorini, F. Pallotti, G. Viale, M. L. Gishizky, G. F. Draetta, and P. P. Di Fiore. 2006. Frequent alterations in the expression of serine/threonine kinases in human cancers. Cancer Res. 668147-8154. - PubMed
-
- Chen, J., L. Li, Y. Zhang, H. Yang, Y. Wei, L. Zhang, X. Liu, and L. Yu. 2006. Interaction of Pin1 with Nek6 and characterization of their expression correlation in Chinese hepatocellular carcinoma patients. Biochem. Biophys. Res. Commun. 3411059-1065. - PubMed
-
- Davies, H., C. Hunter, R. Smith, P. Stephens, C. Greenman, G. Bignell, J. Teague, A. Butler, S. Edkins, C. Stevens, A. Parker, S. O'Meara, T. Avis, S. Barthorpe, L. Brackenbury, G. Buck, J. Clements, J. Cole, E. Dicks, K. Edwards, S. Forbes, M. Gorton, K. Gray, K. Halliday, R. Harrison, K. Hills, J. Hinton, D. Jones, V. Kosmidou, R. Laman, R. Lugg, A. Menzies, J. Perry, R. Petty, K. Raine, R. Shepherd, A. Small, H. Solomon, Y. Stephens, C. Tofts, J. Varian, A. Webb, S. West, S. Widaa, A. Yates, F. Brasseur, C. S. Cooper, A. M. Flanagan, A. Green, M. Knowles, S. Y. Leung, L. H. Looijenga, B. Malkowicz, M. A. Pierotti, B. T. Teh, S. T. Yuen, S. R. Lakhani, D. F. Easton, B. L. Weber, P. Goldstraw, A. G. Nicholson, R. Wooster, M. R. Stratton, and P. A. Futreal. 2005. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. 657591-7595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
