Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia
- PMID: 19414673
- PMCID: PMC2702232
- DOI: 10.1200/JCO.2008.20.2630
Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia
Abstract
Purpose: To conduct a phase II trial in chemoresistant hairy cell leukemia (HCL) with BL22, a recombinant anti-CD22 immunotoxin which showed phase I activity in HCL.
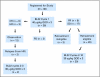
Patients and methods: Eligible patients had relapsed/refractory HCL and needed treatment based on blood counts. Patients were stratified into three groups: response to cladribine less than 1 year, those with a response lasting 1 to 4 years, or no response and uncontrolled infection. Patients received BL22 40 microg/kg every other day for three doses on cycle 1. Those achieving hematologic remission (HR), defined as neutrophils > or = 1,500/mm(3), hemoglobin > or = 11 g/dL, and platelets > or = 100,000/mm(3), were observed. Patients without HR were re-treated at 30 microg/kg every other day for three doses every 4 weeks beginning at least 8 weeks after cycle 1.
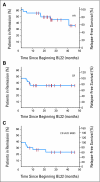
Results: Thirty-six patients were enrolled including 26, nine, and one in groups 1 to 3. The response after one cycle (CR, 25%; PR, 25%) improved when 56% were re-treated (CR, 47%; PR, 25%). CR rate was similar in groups 1 and 2 (P = .7). Twenty-two with baseline spleen height lower than 200 mm had higher CR (64% v 21%; P = .019) and OR rates (95% v 36%; P = .0002) compared to 14 with spleens either absent or higher than 200 mm. The only serious toxicity was reversible grade 3 hemolytic uremic syndrome, not requiring plasmapheresis, in two patients (6%). High neutralizing antibodies were observed in four patients (11%) and prevented re-treatment.
Conclusion: BL22 activity in HCL is confirmed. Best responses to BL22 after cladribine failure are achieved before the patients develop massive splenomegaly or undergo splenectomy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood. 1958;13:609–630. - PubMed
-
- Tallman MS, Hakimian D, Rademaker AW, et al. Relapse of hairy cell leukemia after 2-chlorodeoxyadenosine: Long-term follow-up of the Northwestern University experience. Blood. 1996;88:1954–1959. - PubMed
-
- Saven A, Burian C, Koziol JA, et al. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92:1918–1926. - PubMed
-
- Goodman GR, Burian C, Koziol JA, et al. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol. 2003;21:891–896. - PubMed
-
- Fanta PT, Saven A. Hairy cell leukemia. Cancer Treat Res. 2008;142:193–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

