Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma
- PMID: 19414675
- PMCID: PMC3646306
- DOI: 10.1200/JCO.2008.19.8903
Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma
Abstract
Purpose: To evaluate patient-specific immunotherapy with mitumprotimut-T (idiotype keyhole limpet hemocyanin [Id-KLH]) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in CD20(+) follicular lymphoma.
Patients and methods: Patients with treatment-naive or relapsed/refractory disease achieving a complete response (CR), partial response (PR), or stable disease (SD) with four weekly rituximab infusions were randomly assigned to mitumprotimut-T/GM-CSF or placebo/GM-CSF, with doses given monthly for six doses, every 2 months for six doses, and then every 3 months until disease progression (PD). Randomization was stratified by prior therapy (treatment-naive or relapsed/refractory) and response to rituximab (CR/PR or SD). The primary end point was time to progression (TTP) from randomization.
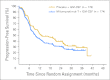
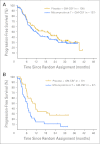
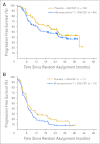
Results: A total of 349 patients were randomly assigned; median age was 54 years, 79% were treatment naive, and 86% had stage III/IV disease. Median TTP was 9.0 months for mitumprotimut-T/GM-CSF and 12.6 months for placebo/GM-CSF (hazard ratio [HR] = 1.384; P = .019). TTP was comparable between the two arms in treatment-naive patients (HR = 1.196; P = .258) and shorter with mitumprotimut-T/GM-CSF in relapsed/refractory disease (HR = 2.265; P = .004). After adjusting for Follicular Lymphoma International Prognostic Index (FLIPI) scores, the difference in TTP between the two arms was no longer significant. Overall objective response rate, rate of response improvement, and duration of response were comparable between the two arms. Toxicity was similar in the two arms; 76% of adverse events were mild or moderate, and 94% of patients had injection site reactions.
Conclusion: TTP was shorter with mitumprotimut-T/GM-CSF compared with placebo/GM-CSF. This difference was possibly due to the imbalance in FLIPI scores.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
A phase 2 trial of immunotherapy with mitumprotimut-T (Id-KLH) and GM-CSF following rituximab in follicular B-cell lymphoma.J Immunother. 2010 Feb-Mar;33(2):178-84. doi: 10.1097/CJI.0b013e3181bfcea1. J Immunother. 2010. PMID: 20145546 Clinical Trial.
-
Active idiotypic vaccination versus control immunotherapy for follicular lymphoma.J Clin Oncol. 2014 Jun 10;32(17):1797-803. doi: 10.1200/JCO.2012.43.9273. Epub 2014 May 5. J Clin Oncol. 2014. PMID: 24799467 Free PMC article. Clinical Trial.
-
Granulocyte-macrophage colony-stimulating factor potentiates rituximab in patients with relapsed follicular lymphoma: results of a phase II study.J Clin Oncol. 2008 Jun 1;26(16):2725-31. doi: 10.1200/JCO.2007.13.7729. Epub 2008 Apr 21. J Clin Oncol. 2008. PMID: 18427151 Free PMC article. Clinical Trial.
-
Therapeutic lymphoma vaccines: importance of T-cell immunity.Expert Rev Vaccines. 2006 Jun;5(3):381-94. doi: 10.1586/14760584.5.3.381. Expert Rev Vaccines. 2006. PMID: 16827622 Review.
-
BiovaxID idiotype vaccination: active immunotherapy for follicular lymphoma.Expert Rev Vaccines. 2007 Jun;6(3):307-17. doi: 10.1586/14760584.6.3.307. Expert Rev Vaccines. 2007. PMID: 17542746 Review.
Cited by
-
Therapeutic vaccines for aggressive B-cell lymphoma.Leuk Lymphoma. 2020 Dec;61(13):3038-3051. doi: 10.1080/10428194.2020.1805113. Epub 2020 Aug 25. Leuk Lymphoma. 2020. PMID: 32840404 Free PMC article.
-
Immunological monitoring of anticancer vaccines in clinical trials.Oncoimmunology. 2013 Aug 1;2(8):e26012. doi: 10.4161/onci.26012. Epub 2013 Aug 23. Oncoimmunology. 2013. PMID: 24083085 Free PMC article. Review.
-
Immune Modulation in Hematologic Malignancies.Semin Oncol. 2015 Aug;42(4):617-25. doi: 10.1053/j.seminoncol.2015.05.009. Epub 2015 Jun 3. Semin Oncol. 2015. PMID: 26320065 Free PMC article. Review.
-
Successes, failures and new perspectives of idiotypic vaccination for B-cell non-Hodgkin lymphomas.Hum Vaccin Immunother. 2013 May;9(5):1078-83. doi: 10.4161/hv.23970. Epub 2013 Feb 13. Hum Vaccin Immunother. 2013. PMID: 23406835 Free PMC article. Review.
-
A vaccine directed to B cells and produced by cell-free protein synthesis generates potent antilymphoma immunity.Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14526-31. doi: 10.1073/pnas.1211018109. Epub 2012 Aug 8. Proc Natl Acad Sci U S A. 2012. PMID: 22875703 Free PMC article.
References
-
- Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. - PubMed
-
- Hsu FJ, Caspar CB, Czerwinski D, et al. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma: Long-term results of a clinical trial. Blood. 1997;89:3129–3135. - PubMed
-
- Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. - PubMed
-
- Redfern CH, Guthrie TH, Bessudo A, et al. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24:3107–3112. - PubMed
-
- Koc ON, Redfern C, Wiernik PH, et al. Continued late conversion to complete remission (CR/CRu) and durability of remission (DUR) in pts with B-cell follicular lymphoma (FL) treated with rituximab followed by mitumprotimut-T (Id-KLH, FavId) immunotherapy. Blood. 2007;110 abstr 3427.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

