TLR signals promote IL-6/IL-17-dependent transplant rejection
- PMID: 19414775
- PMCID: PMC2834528
- DOI: 10.4049/jimmunol.0803842
TLR signals promote IL-6/IL-17-dependent transplant rejection
Abstract
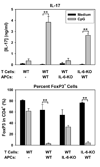
Acute allograft rejection has often been correlated with Th1 differentiation, whereas transplantation tolerance is frequently associated with induction of regulation. The discovery of the Th17 phenotype has prompted its scrutiny in transplant rejection. Although IL-17 has recently been observed in settings of acute allograft rejection and drives rejection in T-bet-deficient mice that have impaired type 1 T cell responses, there is little evidence of its requirement during acute rejection in wild-type animals. We and others have previously shown that TLR9 signaling by exogenous CpG at the time of transplantation is sufficient to abrogate anti-CD154-mediated acceptance of fully mismatched cardiac allografts. In this study, we investigated the mechanism by which acute rejection occurs in this inflammatory context. Our results indicate that CpG targets recipient hemopoietic cells and that its pro-rejection effects correlate both with prevention of anti-CD154-mediated conversion of conventional CD4(+) T cells into induced regulatory T cells and with the expression of IFN-gamma and IL-17 by intragraft CD4(+) T cells. Moreover, the combined elimination of IL-6 and IL-17 signaling abrogated the ability of CpG to promote acute cardiac allograft rejection. Thus, proinflammatory signals at the time of transplantation can change the quality of the effector immune response and reveal a pathogenic function for IL-6 and IL-17 in wild-type recipients.
Figures







References
-
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. - PubMed
-
- Chen Y, Wood KJ. Interleukin-23 and TH17 cells in transplantation immunity: does 23+17 equal rejection? Transplantation. 2007;84:1071–1074. - PubMed
-
- Zhou P, Szot GL, Guo Z, Kim O, He G, Wang J, Grusby MJ, Newell KA, Thistlethwaite JR, Bluestone JA, Alegre ML. Role of STAT4 and STAT6 signaling in allograft rejection and CTLA4-Ig-mediated tolerance. J Immunol. 2000;165:5580–5587. - PubMed
-
- Kishimoto K, Dong VM, Issazadeh S, Fedoseyeva EV, Waaga AM, Yamada A, Sho M, Benichou G, Auchincloss H, Jr, Grusby MJ, Khoury SJ, Sayegh MH. The role of CD154-CD40 versus CD28-B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest. 2000;106:63–72. - PMC - PubMed
-
- Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002;197:322–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

