Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome
- PMID: 19414800
- PMCID: PMC2722440
- DOI: 10.4049/jimmunol.0802696
Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome
Abstract
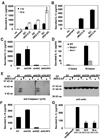
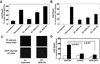
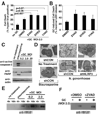
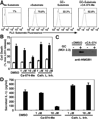
Neisseria gonorrhoeae is a common sexually transmitted pathogen that significantly impacts female fertility, neonatal health, and transmission of HIV worldwide. N. gonorrhoeae usually causes localized inflammation of the urethra and cervix by inducing production of IL-1beta and other inflammatory cytokines. Several NLR (nucleotide-binding domain, leucine-rich repeat) proteins are implicated in the formation of pro-IL-1beta-processing complexes called inflammasomes in response to pathogens. We demonstrate that NLRP3 (cryopyrin, NALP3) is the primary NLR required for IL-1beta/IL-18 secretion in response to N. gonorrhoeae in monocytes. We also show that N. gonorrhoeae infection promotes NLRP3-dependent monocytic cell death via pyronecrosis, a recently described pathway with morphological features of necrosis, including release of the strong inflammatory mediator HMBG1. Additionally, N. gonorrhoeae activates the cysteine protease cathepsin B as measured by the breakdown of a cathepsin B substrate. Inhibition of cathepsin B shows that this protease is an apical controlling step in the downstream activities of NLRP3 including IL-1beta production, pyronecrosis, and HMGB1 release. Nonpathogenic Neisseria strains (Neisseria cinerea and Neisseria flavescens) do not activate NLRP3 as robustly as N. gonorrhoeae. Conditioned medium from N. gonorrhoeae contains factors capable of initiating the NLRP3-mediated signaling events. Isolated N. gonorrhoeae lipooligosaccharide, a known virulence factor from this bacterium that is elaborated from the bacterium in the form of outer membrane blebs, activates both NLRP3-induced IL-1beta secretion and pyronecrosis. Our findings indicate that activation of NLRP3-mediated inflammatory response pathways is an important venue associated with host response and pathogenesis of N. gonorrhoeae.
Figures


References
-
- Tapsall JW. Antibiotic resistance in Neisseria gonorrhoeae. Clin Infect Dis. 2005;41 Suppl 4:S263–S268. - PubMed
-
- Fox KK, Thomas JC, Weiner DH, Davis RH, Sparling PF, Cohen MS. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am J Epidemiol. 1999;149:353–358. - PubMed
-
- Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. - PubMed
-
- Ramsey KH, Schneider H, Kuschner RA, Trofa AF, Cross AS, Deal CD. Inflammatory cytokine response to experimental human infection with Neisseria gonorrhoeae. Ann N Y Acad Sci. 1994;730:322–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

