Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens
- PMID: 19414814
- PMCID: PMC2711537
- DOI: 10.4049/jimmunol.0900317
Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens
Erratum in
- J Immunol. 2009 Dec 1;183(11):7611. Haut, Larissa H [added]
Abstract
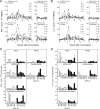
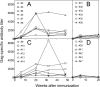
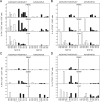
In the phase IIb STEP trial an HIV-1 vaccine based on adenovirus (Ad) vectors of the human serotype 5 (AdHu5) not only failed to induce protection but also increased susceptibility to HIV-1 infection in individuals with preexisting neutralizing Abs against AdHu5. The mechanisms underlying the increased HIV-1 acquisition rates have not yet been elucidated. Furthermore, it remains unclear if the lack of the vaccine's efficacy reflects a failure of the concept of T cell-mediated protection against HIV-1 or a product failure of the vaccine. Here, we compared two vaccine regimens based on sequential use of AdHu5 vectors or two different chimpanzee-derived Ad vectors in rhesus macaques that were AdHu5 seropositive or seronegative at the onset of vaccination. Our results show that heterologous booster immunizations with the chimpanzee-derived Ad vectors induced higher T and B cell responses than did repeated immunizations with the AdHu5 vector, especially in AdHu5-preexposed macaques.
Figures


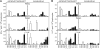



References
-
- Robinson HL. HIV/AIDS vaccines: 2007. Clin. Pharmacol. Ther. 2007;82:686–93. - PubMed
-
- Steinbrook R. One Step Forward, Two Steps Back -- Will There Ever Be an AIDS Vaccine? N. Engl. J. Med. 2007;357:2653–2655. - PubMed
-
- Hanke T. STEP trial and HIV-1 vaccines inducing T-cell responses. Expert Rev. Vaccines. 2008;7:303–9. - PubMed
-
- Moore JP, Klasse PJ, Dolan MJ, Ahuja SK. AIDS/HIV. A STEP into darkness or light? Science. 2008;320:753–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

