Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers
- PMID: 19414990
- PMCID: PMC3652655
- DOI: 10.1097/QAD.0b013e328329f97d
Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers
Abstract
Objective: To determine the spectrum of antiviral antibodies in HIV-1-infected individuals in whom viral replication is spontaneously undetectable, termed HIV controllers (HICs).
Design: Multicenter French trial ANRS EP36 studying the viral control in HICs.
Methods: Neutralizing Antibody (nAb) activities (neutralization assay, competition with broadly reactive monoclonal antibodies, and reactivity against the viral MPER gp41 region), FcgammaR-mediated antiviral activities, antibody-dependent cell cytotoxicity (ADCC), as well as autoantibody levels, were quantified in plasma from 22 controllers and from viremic individuals. The levels of these different antibody responses and HIV-specific CD8 T cell responses quantified by enzyme-linked immunosorbent spot (ELISPOT) IFNgamma assay were compared in each controller.
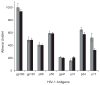
Results: The levels of antibody against the gp120 CD4 binding site, gp41, as well as Env epitopes near to the sites bound by broadly nAbs 2F5 and 1b12 were not different between HICs and viremic individuals. We did not find significant autoantibody levels in HICs. The magnitude and breadth of nAbs were heterogeneous in HICs but lower than in viremic individuals. The levels of nAbs using FcgammaR-mediated assay inhibition were similar in both groups. Regardless of the type of antibody tested, there was no correlation with HIV-specific CD8 T cell responses. ADCC was detectable in all controllers tested and was significantly higher than in viremic individuals (P < 0.0002).
Conclusion: There was no single anti-HIV-1 antibody specificity that was a clear correlate of immunity in controllers. Rather, for most antibody types, controllers had the same or lower levels of nAbs than viremic individuals, with the possible exception of ADCC antibodies.
Figures




References
-
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. - PubMed
-
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

