Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA
- PMID: 19415341
- PMCID: PMC3911883
- DOI: 10.1007/s00520-009-0642-2
Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA
Abstract
Purpose: This pilot trial sought to investigate whether any of three doses of American ginseng (Panax quinquefolius) might help cancer-related fatigue. A secondary aim was to evaluate toxicity.
Methods: Eligible adults with cancer were randomized in a double-blind manner, to receive American ginseng in doses of 750, 1,000, or 2,000 mg/day or placebo given in twice daily dosing over 8 weeks. Outcome measures included the Brief Fatigue Inventory, vitality subscale of the Medical Outcome Scale Short Form-36 (SF-36), and the Global Impression of Benefit Scale at 4 and 8 weeks.
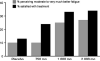
Results: Two hundred ninety patients were accrued to this trial. Nonsignificant trends for all outcomes were seen in favor of the 1,000- and 2,000-mg/day doses of American ginseng. Area under the curve analysis of activity interference from the Brief Fatigue Inventory was 460-467 in the placebo group and 750 mg/day group versus 480-551 in the 1,000- and 2,000-mg/day arms, respectively. Change from baseline in the vitality subscale of the SF-36 was 7.3-7.8 in the placebo and the 750-mg/day arm, versus 10.5-14.6 in the 1,000- and 2,000-mg/day arms. Over twice as many patients on ginseng perceived a benefit and were satisfied with treatment over those on placebo. There were no significant differences in any measured toxicities between any of the arms.
Conclusion: There appears to be some activity and tolerable toxicity at 1,000-2,000 mg/day doses of American ginseng with regard to cancer-related fatigue. Thus, further study of American ginseng is warranted.
Figures
References
-
- NCCN Cancer Related Fatigue Clinical Practice Guidelines in Oncology V.1. 2003 http://www.nccn.org/ - PubMed
-
- Fulton C, Knowles G. Cancer fatigue. Eur J Cancer Care (Engl) 2000;9(3):167–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA035267/CA/NCI NIH HHS/United States
- U10 CA052352/CA/NCI NIH HHS/United States
- CA-35090/CA/NCI NIH HHS/United States
- U10 CA060276/CA/NCI NIH HHS/United States
- CA-52352/CA/NCI NIH HHS/United States
- CA-35195/CA/NCI NIH HHS/United States
- U10 CA037404/CA/NCI NIH HHS/United States
- CA-25224/CA/NCI NIH HHS/United States
- CA-60276/CA/NCI NIH HHS/United States
- U10 CA035113/CA/NCI NIH HHS/United States
- CA-35415/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
- U10 CA037417/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA035269/CA/NCI NIH HHS/United States
- CA-37404/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- U10 CA035448/CA/NCI NIH HHS/United States
- U10 CA063848/CA/NCI NIH HHS/United States
- U10 CA035195/CA/NCI NIH HHS/United States
- CA-35103/CA/NCI NIH HHS/United States
- CA-35113/CA/NCI NIH HHS/United States
- U10 CA035101/CA/NCI NIH HHS/United States
- CA-63848/CA/NCI NIH HHS/United States
- U10 CA035415/CA/NCI NIH HHS/United States
- U10 CA063849/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- CA-37417/CA/NCI NIH HHS/United States
- CA-35269/CA/NCI NIH HHS/United States
- CA-35448/CA/NCI NIH HHS/United States
- CA-35101/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- CA-35267/CA/NCI NIH HHS/United States
- CA-63849/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical