Regulation and function of proline oxidase under nutrient stress
- PMID: 19415679
- PMCID: PMC2801574
- DOI: 10.1002/jcb.22174
Regulation and function of proline oxidase under nutrient stress
Abstract
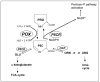
Under conditions of nutrient stress, cells switch to a survival mode catabolizing cellular and tissue constituents for energy. Proline metabolism is especially important in nutrient stress because proline is readily available from the breakdown of extracellular matrix (ECM), and the degradation of proline through the proline cycle initiated by proline oxidase (POX), a mitochondrial inner membrane enzyme, can generate ATP. This degradative pathway generates glutamate and alpha-ketoglutarate, products that can play an anaplerotic role for the TCA cycle. In addition the proline cycle is in a metabolic interlock with the pentose phosphate pathway providing another bioenergetic mechanism. Herein we have investigated the role of proline metabolism in conditions of nutrient stress in the RKO colorectal cancer cell line. The induction of stress either by glucose withdrawal or by treatment with rapamycin, stimulated degradation of proline and increased POX catalytic activity. Under these conditions POX was responsible, at least in part, for maintenance of ATP levels. Activation of AMP-activated protein kinase (AMPK), the cellular energy sensor, by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), also markedly upregulated POX and increased POX-dependent ATP levels, further supporting its role during stress. Glucose deprivation increased intracellular proline levels, and expression of POX activated the pentose phosphate pathway. Together, these results suggest that the induction of proline cycle under conditions of nutrient stress may be a mechanism by which cells switch to a catabolic mode for maintaining cellular energy levels.
2009 Wiley-Liss, Inc.
Figures









References
-
- Bates L. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207.
-
- Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–4173. - PubMed
-
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

