Identification of gastric cancer stem cells using the cell surface marker CD44
- PMID: 19415765
- PMCID: PMC2746367
- DOI: 10.1002/stem.30
Identification of gastric cancer stem cells using the cell surface marker CD44
Abstract
Cancer stem cells (CSCs) have been defined as a unique subpopulation in tumors that possess the ability to initiate tumor growth and sustain tumor self-renewal. Although the evidence has been provided to support the existence of CSCs in various solid tumors, the identity of gastric CSCs has not been reported. In this study, we have identified gastric cancer-initiating cells from a panel of human gastric cancer cell lines using cell surface marker CD44. Among six gastric cancer cell lines, three lines MKN-45, MKN-74, and NCI-N87 had a sizeable subpopulation of CD44(+) cells, and these cells showed spheroid colony formation in serum-free media in vitro as well as tumorigenic ability when injected into stomach and skin of severe combined immunodeficient (SCID) mice in vivo. The CD44(+) gastric cancer cells showed the stem cell properties of self-renewal and the ability to form differentiated progeny and gave rise to CD44(-) cells. CD44 knockdown by short hairpin RNA resulted in much reduced spheroid colony formation and smaller tumor production in SCID mice, and the CD44(-) populations had significantly reduced tumorigenic ability in vitro and in vivo. Other potential CSC markers, such as CD24, CD133, CD166, stage-specific embryonic antigen-1 (SSEA-1), and SSEA-4, or sorting for side population did not show any correlation with tumorigenicity in vitro or in vivo. The CD44(+) gastric cancer cells showed increased resistance for chemotherapy- or radiation-induced cell death. These results support the existence of gastric CSCs and may provide novel approaches to the diagnosis and treatment of gastric cancer.
Conflict of interest statement
Figures
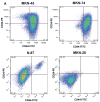

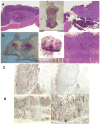
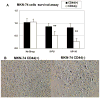

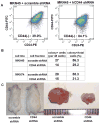


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Houghton J, Morozov A, Smirnova I, et al. Stem cells and cancer. Semin Cancer Biol. 2007;17:191–203. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

