Expression of receptor activator of nuclear factor-kappaB ligand by B cells in response to oral bacteria
- PMID: 19416447
- PMCID: PMC2692067
- DOI: 10.1111/j.1399-302X.2008.00494.x
Expression of receptor activator of nuclear factor-kappaB ligand by B cells in response to oral bacteria
Abstract
Introduction: We investigated receptor activator of nuclear factor-kappaB ligand (RANKL) expression by B lymphocytes during early and late aspects of the immune response to Aggregatibacter actinomycetemcomitans, a gram-negative, anaerobic bacterium associated with aggressive periodontal disease.
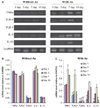
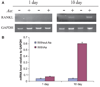
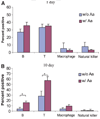
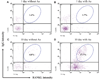
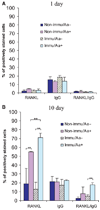
Methods: Expression of messenger RNA transcripts (tumor necrosis factor-alpha, Toll-like receptors 4 and 9, interleukins 4 and 10, and RANKL) involved in early (1-day) and late (10-day) responses in cultured rat splenocytes was examined by reverse transcription-polymerase chain reaction (RT-PCR). The immune cell distribution (T, B, and natural killer cells and macrophages) in cultured rat splenocytes and RANKL expression in B cells were determined by flow cytometric analyses. B-cell capacity for induction of osteoclast differentiation was evaluated by coculture with RAW 264.7 cells followed by a tartrate-resistant acid phosphatase (TRAP) activity assay.
Results: The expression levels of interleukins 4 and 10 in cultured cells were not changed in the presence of A. actinomycetemcomitans until cultured for 3 days, and peaked after 7 days. After culture for 10 days, the percentages of B and T cells, the overall RANKL messenger RNA transcripts, and the percentage of RANKL-expressing immunoglobulin G-positive cells were significantly increased in the presence of A. actinomycetemcomitans. These increases were considerably greater in cells isolated from A. actinomycetemcomitans-immunized animals than from non-immunized animals. RAW 264.7 cells demonstrated significantly increased TRAP activity when cocultured with B cells from A. actinomycetemcomitans-immunized animals. The addition of human osteoprotegerin-Fc to the culture significantly diminished such increases.
Conclusion: This study suggests that B-lymphocyte involvement in the immune response to A. actinomycetemcomitans through upregulation of RANKL expression potentially contribute to bone resorption in periodontal disease.
Figures


References
-
- Taubman MA, Yoshie H, Ebersole JL, Smith DJ, Olson CL. Host response in experimental periodontal disease. J Dent Res. 1984;63:455–460. - PubMed
-
- Stoufi ED, Taubman MA, Ebersole JL, Smith DJ, Stashenko PP. Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. J Clin Immunol. 1987;7:235–245. - PubMed
-
- Seymour GJ, Powell RN, Cole KL, et al. Experimental gingivitis in humans. A histochemical and immunological characterization of the lymphoid cell subpopulations. J Periodontal Res. 1983;18:375–385. - PubMed
-
- Malberg K, Molle A, Streuer D, Gangler P. Determination of lymphocyte populations and subpopulations extracted from chronically inflamed human periodontal tissues. J Clin Periodontol. 1992;19:155–158. - PubMed
-
- Tew J, Engel D, Mangan D. Polyclonal B-cell activation in periodontitis. J Periodontal Res. 1989;24:225–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

