Cyclin D1 fine-tunes the neurogenic output of embryonic retinal progenitor cells
- PMID: 19416500
- PMCID: PMC2694796
- DOI: 10.1186/1749-8104-4-15
Cyclin D1 fine-tunes the neurogenic output of embryonic retinal progenitor cells
Abstract
Background: Maintaining the correct balance of proliferation versus differentiation in retinal progenitor cells (RPCs) is essential for proper development of the retina. The cell cycle regulator cyclin D1 is expressed in RPCs, and mice with a targeted null allele at the cyclin D1 locus (Ccnd1-/-) have microphthalmia and hypocellular retinas, the latter phenotype attributed to reduced RPC proliferation and increased photoreceptor cell death during the postnatal period. How cyclin D1 influences RPC behavior, especially during the embryonic period, is unclear.
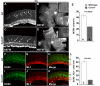
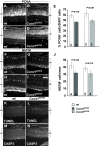
Results: In this study, we show that embryonic RPCs lacking cyclin D1 progress through the cell cycle at a slower rate and exit the cell cycle at a faster rate. Consistent with enhanced cell cycle exit, the relative proportions of cell types born in the embryonic period, such as retinal ganglion cells and photoreceptor cells, are increased. Unexpectedly, cyclin D1 deficiency decreases the proportions of other early born retinal neurons, namely horizontal cells and specific amacrine cell types. We also found that the laminar positioning of horizontal cells and other cell types is altered in the absence of cyclin D1. Genetically replacing cyclin D1 with cyclin D2 is not efficient at correcting the phenotypes due to the cyclin D1 deficiency, which suggests the D-cyclins are not fully redundant. Replacement with cyclin E or inactivation of cyclin-dependent kinase inhibitor p27Kip1 restores the balance of RPCs and retinal cell types to more normal distributions, which suggests that regulation of the retinoblastoma pathway is an important function for cyclin D1 during embryonic retinal development.
Conclusion: Our findings show that cyclin D1 has important roles in RPC cell cycle regulation and retinal histogenesis. The reduction in the RPC population due to a longer cell cycle time and to an enhanced rate of cell cycle exit are likely to be the primary factors driving retinal hypocellularity and altered output of precursor populations in the embryonic Ccnd1-/- retina.
Figures








Similar articles
-
Cyclin D1 inactivation extends proliferation and alters histogenesis in the postnatal mouse retina.Dev Dyn. 2012 May;241(5):941-52. doi: 10.1002/dvdy.23782. Dev Dyn. 2012. PMID: 22434780 Free PMC article.
-
Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors.Neural Dev. 2009 Aug 17;4:32. doi: 10.1186/1749-8104-4-32. Neural Dev. 2009. PMID: 19686589 Free PMC article.
-
Mutual antagonism of the paired-type homeobox genes, vsx2 and dmbx1, regulates retinal progenitor cell cycle exit upstream of ccnd1 expression.Dev Biol. 2015 Jun 15;402(2):216-28. doi: 10.1016/j.ydbio.2015.03.020. Epub 2015 Apr 11. Dev Biol. 2015. PMID: 25872183
-
Cell-intrinsic regulators of proliferation in vertebrate retinal progenitors.Semin Cell Dev Biol. 2004 Feb;15(1):63-74. doi: 10.1016/j.semcdb.2003.09.001. Semin Cell Dev Biol. 2004. PMID: 15036209 Review.
-
Neocortical neurogenesis: morphogenetic gradients and beyond.Trends Neurosci. 2009 Aug;32(8):443-50. doi: 10.1016/j.tins.2009.05.003. Epub 2009 Jul 25. Trends Neurosci. 2009. PMID: 19635637 Free PMC article. Review.
Cited by
-
Targeted ablation of CRB1 and CRB2 in retinal progenitor cells mimics Leber congenital amaurosis.PLoS Genet. 2013;9(12):e1003976. doi: 10.1371/journal.pgen.1003976. Epub 2013 Dec 5. PLoS Genet. 2013. PMID: 24339791 Free PMC article.
-
Proliferation potential of Müller glia after retinal damage varies between mouse strains.PLoS One. 2014 Apr 18;9(4):e94556. doi: 10.1371/journal.pone.0094556. eCollection 2014. PLoS One. 2014. PMID: 24747725 Free PMC article.
-
Cyclins and cell cycle control in cancer and disease.Genes Cancer. 2012 Nov;3(11-12):649-57. doi: 10.1177/1947601913479022. Genes Cancer. 2012. PMID: 23634253 Free PMC article.
-
Vsx2 controls eye organogenesis and retinal progenitor identity via homeodomain and non-homeodomain residues required for high affinity DNA binding.PLoS Genet. 2012 Sep;8(9):e1002924. doi: 10.1371/journal.pgen.1002924. Epub 2012 Sep 20. PLoS Genet. 2012. PMID: 23028343 Free PMC article.
-
Single-cell analyses reveal transient retinal progenitor cells in the ciliary margin of developing human retina.Nat Commun. 2024 Apr 26;15(1):3567. doi: 10.1038/s41467-024-47933-x. Nat Commun. 2024. PMID: 38670973 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

