Microsomal glutathione transferase 1 exhibits one-third-of-the-sites-reactivity towards glutathione
- PMID: 19416719
- PMCID: PMC2996728
- DOI: 10.1016/j.abb.2009.04.009
Microsomal glutathione transferase 1 exhibits one-third-of-the-sites-reactivity towards glutathione
Abstract
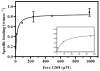
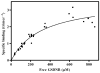
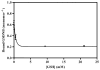
The trimeric membrane protein microsomal glutathione transferase 1 (MGST1) possesses glutathione transferase and peroxidase activity. Previous data indicated one active site/trimer whereas structural data suggests three GSH-binding sites. Here we have determined ligand interactions of MGST1 by several techniques. Nanoelectrospray mass spectrometry of native MGST1 revealed binding of three GSH molecules/trimer and equilibrium dialysis showed three product molecules/trimer (K(d)=320+/-50 microM). All three product molecules could be competed out with GSH. Reinvestigation of GSH-binding showed one high affinity site per trimer, consistent with earlier data. Using single turnover stopped flow kinetic measurements, K(d) could be determined for a low affinity GSH-binding site (2.5+/-0.5 mM). Thus we can reconcile previous observations and show here that MGST1 contains three active sites with different affinities for GSH and that only the high affinity site is catalytically competent.
Figures



References
-
- Rinaldi R, Eliasson E, Swedmark S, Morgenstern R. Drug Metab Dispos. 2002;30:1053–1058. - PubMed
-
- Busenlehner LS, Codreanu SG, Holm PJ, Bhakat P, Hebert H, Morgenstern R, Armstrong RN. Biochemistry. 2004;43:11145–11152. - PubMed
-
- Hebert H, Schmidt-Krey I, Morgenstern R, Murata K, Hirai T, Mitsuoka K, Fujiyoshi Y. J Mol Biol. 1997;271:751–758. - PubMed
-
- Holm PJ, Bhakat P, Jegerschöld C, Gyobu N, Mitsuoka K, Fujiyoshi Y, Morgenstern R, Hebert H. J Mol Biol. 2006;360:934–945. - PubMed
-
- Holm PJ, Morgenstern R, Hebert H. Biochim Biophys Acta. 2002;1594:276–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

