The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity
- PMID: 19416816
- PMCID: PMC2688863
- DOI: 10.1073/pnas.0812808106
The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity
Abstract
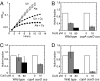
Excess copper is poisonous to all forms of life, and copper overloading is responsible for several human pathologic processes. The primary mechanisms of toxicity are unknown. In this study, mutants of Escherichia coli that lack copper homeostatic systems (copA cueO cus) were used to identify intracellular targets and to test the hypothesis that toxicity involves the action of reactive oxygen species. Low micromolar levels of copper were sufficient to inhibit the growth of both WT and mutant strains. The addition of branched-chain amino acids restored growth, indicating that copper blocks their biosynthesis. Indeed, copper treatment rapidly inactivated isopropylmalate dehydratase, an iron-sulfur cluster enzyme in this pathway. Other enzymes in this iron-sulfur dehydratase family were similarly affected. Inactivation did not require oxygen, in vivo or with purified enzyme. Damage occurred concomitant with the displacement of iron atoms from the solvent-exposed cluster, suggesting that Cu(I) damages these proteins by liganding to the coordinating sulfur atoms. Copper efflux by dedicated export systems, chelation by glutathione, and cluster repair by assembly systems all enhance the resistance of cells to this metal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
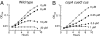



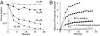

References
-
- Muller T, Feichtinger H, Berger H, Muller W. Endemic Tyrolean infantile cirrhosis: an ecogenetic disorder. Lancet. 1996;347:877–880. - PubMed
-
- Tanner MS, et al. Increased hepatic copper concentration in Indian childhood cirrhosis. Lancet. 1979;8128:1203–1205. - PubMed
-
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. - PubMed
-
- Tanzi RE, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

