MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution
- PMID: 19416870
- PMCID: PMC2688861
- DOI: 10.1073/pnas.0903196106
MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution
Abstract
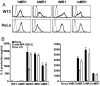
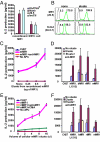
Several nonclassical major histocompatibilty antigens (class Ib molecules) have emerged as key players in the early immune response to pathogens or stress. Class Ib molecules activate subsets of T cells that mount effector responses before the adaptive immune system, and thus are called innate T cells. MR1 is a novel class Ib molecule with properties highly suggestive of its regulation of mucosal immunity. The Mr1 gene is evolutionarily conserved, is non-Mhc linked, and controls the development of mucosal-associated invariant T (MAIT) cells. MAIT cells preferentially reside in the gut, and their development is dependent on commensal microbiota. Although these properties suggest that MAIT cells function as innate T cells in the mucosa, this has been difficult to test, due to the (i) paucity of MAIT cells that display MR1-specific activation in vitro and (ii) lack of knowledge of whether or not MR1 presents antigen. Here we show that both mouse and human MAIT cells display a high level of cross-reactivity on mammalian MR1 orthologs, but with differences consistent with limited ligand discrimination. Furthermore, acid eluates from recombinant or cellular MR1 proteins enhance MAIT cell activation in an MR1-specific and cross-species manner. Our findings demonstrate that the presentation pathway of MR1 to MAIT cells is highly evolutionarily conserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
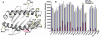
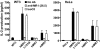

References
-
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. - PubMed
-
- Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. - PubMed
-
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. - PubMed
-
- Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: Innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. - PubMed
-
- Colmone A, Wang CR. H2–M3–restricted T cell response to infection. Microbes Infect. 2006;8:2277–2283. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

