An age-related homeostasis mechanism is essential for spontaneous amelioration of hemophilia B Leyden
- PMID: 19416882
- PMCID: PMC2674395
- DOI: 10.1073/pnas.0902191106
An age-related homeostasis mechanism is essential for spontaneous amelioration of hemophilia B Leyden
Abstract
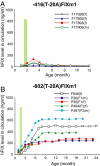
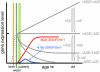
Regulation of age-related changes in gene expression underlies many diseases. We previously discovered the first puberty-onset gene switch, the age-related stability element (ASE)/age-related increase element (AIE)-mediated genetic mechanism for age-related gene regulation. Here, we report that this mechanism underlies the mysterious puberty-onset amelioration of abnormal bleeding seen in hemophilia B Leyden. Transgenic mice robustly mimicking the Leyden phenotype were constructed. Analysis of these animals indicated that ASE plays a central role in the puberty-onset amelioration of the disease. Human factor IX expression in these animals was reproducibly nullified by hypophysectomy, but nearly fully restored by administration of growth hormone, being consistent with the observed sex-independent recovery of factor IX expression. Ets1 was identified as the specific liver nuclear protein binding only to the functional ASE, G/CAGGAAG, and not to other Ets consensus elements. This study demonstrates the clinical relevance of the first discovered puberty-onset gene switch, the ASE/AIE-mediated regulatory mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
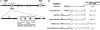
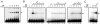

References
-
- Kurachi S, Deyashiki Y, Takeshita J, Kurachi K. Genetic mechanisms of age regulation of human blood coagulation factor IX. Science. 1999;285:739–743. - PubMed
-
- Zhang K, Kurachi S, Kurachi K. Genetic mechanisms of age regulation of protein C and blood coagulation. J Biol Chem. 2002;277:4532–4540. - PubMed
-
- Kurachi K, Kurachi S. Molecular mechanisms of age-related regulation of genes. J Thromb Haemost. 2005;3:909–914. - PubMed
-
- Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361:1801–1809. - PubMed
-
- Terwiel JP, Veltkamp JJ, Bertina RM, van der Linden IK, van Tilburg NH. Coagulation factors in the premature infant born after about 32 weeks of gestation. Biol Neonate. 1985;47:9–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

