Identification and structural basis of the reaction catalyzed by CYP121, an essential cytochrome P450 in Mycobacterium tuberculosis
- PMID: 19416919
- PMCID: PMC2678619
- DOI: 10.1073/pnas.0812191106
Identification and structural basis of the reaction catalyzed by CYP121, an essential cytochrome P450 in Mycobacterium tuberculosis
Abstract
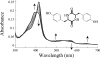
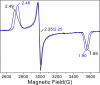
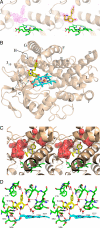
The gene encoding the cytochrome P450 CYP121 is essential for Mycobacterium tuberculosis. However, the CYP121 catalytic activity remains unknown. Here, we show that the cyclodipeptide cyclo(l-Tyr-l-Tyr) (cYY) binds to CYP121, and is efficiently converted into a single major product in a CYP121 activity assay containing spinach ferredoxin and ferredoxin reductase. NMR spectroscopy analysis of the reaction product shows that CYP121 catalyzes the formation of an intramolecular C-C bond between 2 tyrosyl carbon atoms of cYY resulting in a novel chemical entity. The X-ray structure of cYY-bound CYP121, solved at high resolution (1.4 A), reveals one cYY molecule with full occupancy in the large active site cavity. One cYY tyrosyl approaches the heme and establishes a specific H-bonding network with Ser-237, Gln-385, Arg-386, and 3 water molecules, including the sixth iron ligand. These observations are consistent with low temperature EPR spectra of cYY-bound CYP121 showing a change in the heme environment with the persistence of the sixth heme iron ligand. As the carbon atoms involved in the final C-C coupling are located 5.4 A apart according to the CYP121-cYY complex crystal structure, we propose that C-C coupling is concomitant with substrate tyrosyl movements. This study provides insight into the catalytic activity, mechanism, and biological function of CYP121. Also, it provides clues for rational design of putative CYP121 substrate-based antimycobacterial agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Corbett EL, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. - PubMed
-
- Cole ST, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. - PubMed
-
- Isin EM, Guengerich FP. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim Biophys Acta. 2007;1770:314–329. - PubMed
-
- McLean KJ, et al. The preponderance of P450s in the Mycobacterium tuberculosis genome. Trends Microbiol. 2006;14:220–228. - PubMed
-
- Ahmad Z, Sharma S, Khuller GK. Azole antifungals as novel chemotherapeutic agents against murine tuberculosis. FEMS Microbiol Lett. 2006;261:181–186. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

