The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice
- PMID: 19417056
- PMCID: PMC2700534
- DOI: 10.1105/tpc.108.065391
The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice
Abstract
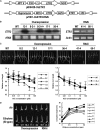
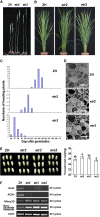
Ethylene regulates multiple aspects of plant growth and development in dicotyledonous plants; however, its roles in monocotyledonous plants are poorly known. Here, we characterized a subfamily II ethylene receptor, ETHYLENE RESPONSE2 (ETR2), in rice (Oryza sativa). The ETR2 receptor with a diverged His kinase domain is a Ser/Thr kinase, but not a His kinase, and can phosphorylate its receiver domain. Mutation of the N box of the kinase domain abolished the kinase activity of ETR2. Overexpression of ETR2 in transgenic rice plants reduced ethylene sensitivity and delayed floral transition. Conversely, RNA interference (RNAi) plants exhibited early flowering and the ETR2 T-DNA insertion mutant etr2 showed enhanced ethylene sensitivity and early flowering. The effective panicles and seed-setting rate were reduced in the ETR2-overexpressing plants, while thousand-seed weight was substantially enhanced in both the ETR2-RNAi plants and the etr2 mutant compared with controls. Starch granules accumulated in the internodes of the ETR2-overexpressing plants, but not in the etr2 mutant. The GIGANTEA and TERMINAL FLOWER1/CENTRORADIALIS homolog (RCN1) that cause delayed flowering were upregulated in ETR2-overexpressing plants but downregulated in the etr2 mutant. Conversely, the alpha-amylase gene RAmy3D was suppressed in ETR2-overexpressing plants but enhanced in the etr2 mutant. Thus, ETR2 may delay flowering and cause starch accumulation in stems by regulating downstream genes.
Figures







References
-
- Abeles, F.B., Morgan, P.W., and Saltveit, M.E., Jr. (1992). Ethylene in Plant Biology, 2nd ed. (San Diego, CA: Academic Press).
-
- Achard, P., Baghour, M., Chapple, A., Hedden, P., Van der Straeten, D., Genschik, P., Moritz, T., and Harberd, N.P. (2007). The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc. Natl. Acad. Sci. USA 104 6484–6489. - PMC - PubMed
-
- Aida, M., Ishida, T., and Tasaka, M. (1999). Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis, interaction among the CUP-SHAPED COYLEDON and SHOOT MERISTEMLESS genes. Development 126 1563–1570. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

