Amino acid containing thapsigargin analogues deplete androgen receptor protein via synthesis inhibition and induce the death of prostate cancer cells
- PMID: 19417145
- PMCID: PMC3383025
- DOI: 10.1158/1535-7163.MCT-08-1136
Amino acid containing thapsigargin analogues deplete androgen receptor protein via synthesis inhibition and induce the death of prostate cancer cells
Abstract
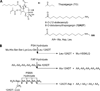
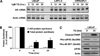
There are quantitative and/or qualitative mechanisms allowing androgen receptor (AR) growth signaling in androgen ablation refractory prostate cancer cells. Regardless of the mechanism, agents that deplete AR protein expression prevent such AR growth signaling. Thapsigargin (TG) is a highly cell-penetrant sequiterpene-lactone that once inside cells inhibits (IC(50), ∼ 10 nmol/L) critically important housekeeping SERCA 2b calcium pumps in the endoplasmic reticulum. Using a series of five genetically diverse androgen ablation refractory human prostate cancer lines (LNCaP, LAPC-4, VCaP, MDA-PCa-2b, and CWR22Rv1), TG inhibition of SERCA pumps consistently results in depletion of the endoplasmic reticulum Ca(+2) coupled with μmol/L elevation in the intracellular free Ca(+2) initiating a molecular cascade that: (a) inhibits Cap-dependent AR protein synthesis resulting in 90% depletion of AR protein by 24 hours of TG exposure, (b) arrests the cells in G(0), and (c) induces their apoptotic death. Unfortunately, due to its highly lipophilic nature, TG is not deliverable as a systemic agent without host toxicity. Therefore, TG analogues containing amino acids were developed, which retain ability to deplete AR protein and induce cell death and which can be covalently linked to peptide carriers producing water soluble prodrugs for systemic delivery. Specific amino acid sequences are used to restrict the liberation of cytotoxic amino acid containing TG analogues from the peptide prodrug by prostate-specific proteases, such as prostate-specific antigen and prostate-specific membrane antigen, or cancer-specific proteases, such as fibroblast activation protein, so that toxicity of these prodrugs is selectively targeted to metastatic sites of prostate cancer. Based on these results, these prodrugs are undergoing clinical development.
Conflict of interest statement
J.T. Isaacs and S.R. Denmeade: financial interest, Genspera, Inc., and Protox Therapeutics, Inc. J.T. Isaacs: grant support, Active Biotech. No other potential conflicts of interest were disclosed.
Figures


Similar articles
-
Pharmacological inhibition of androgen receptor expression induces cell death in prostate cancer cells.Cell Mol Life Sci. 2020 Nov;77(22):4663-4673. doi: 10.1007/s00018-019-03429-2. Epub 2020 Jan 1. Cell Mol Life Sci. 2020. PMID: 31894360 Free PMC article.
-
Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models.Prostate. 2003 Mar 1;54(4):249-57. doi: 10.1002/pros.10199. Prostate. 2003. PMID: 12539223
-
Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells.Prostate. 2000 Jun 1;43(4):303-17. doi: 10.1002/1097-0045(20000601)43:4<303::aid-pros10>3.0.co;2-v. Prostate. 2000. PMID: 10861750
-
Mipsagargin: The Beginning-Not the End-of Thapsigargin Prodrug-Based Cancer Therapeutics.Molecules. 2021 Dec 9;26(24):7469. doi: 10.3390/molecules26247469. Molecules. 2021. PMID: 34946547 Free PMC article. Review.
-
The SERCA pump as a therapeutic target: making a "smart bomb" for prostate cancer.Cancer Biol Ther. 2005 Jan;4(1):14-22. doi: 10.4161/cbt.4.1.1505. Epub 2005 Jan 23. Cancer Biol Ther. 2005. PMID: 15662118 Review.
Cited by
-
From Plant to Patient: Thapsigargin, a Tool for Understanding Natural Product Chemistry, Total Syntheses, Biosynthesis, Taxonomy, ATPases, Cell Death, and Drug Development.Prog Chem Org Nat Prod. 2021;115:59-114. doi: 10.1007/978-3-030-64853-4_2. Prog Chem Org Nat Prod. 2021. PMID: 33797641
-
The stress response mediator ATF3 represses androgen signaling by binding the androgen receptor.Mol Cell Biol. 2012 Aug;32(16):3190-202. doi: 10.1128/MCB.00159-12. Epub 2012 Jun 4. Mol Cell Biol. 2012. PMID: 22665497 Free PMC article.
-
Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response.J Biol Chem. 2017 Dec 1;292(48):19656-19673. doi: 10.1074/jbc.M117.796920. Epub 2017 Sep 29. J Biol Chem. 2017. PMID: 28972171 Free PMC article.
-
Consequence of evolutionary loss of seasonal breeding by humans for prostate cancer chemoprevention.Am J Clin Exp Urol. 2023 Jun 15;11(3):194-205. eCollection 2023. Am J Clin Exp Urol. 2023. PMID: 37441442 Free PMC article. Review.
-
Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug.J Natl Cancer Inst. 2012 Sep 5;104(17):1320-34. doi: 10.1093/jnci/djs336. Epub 2012 Aug 21. J Natl Cancer Inst. 2012. PMID: 22911669 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy programd. Cancer Res. 2004;64:9209–9216. - PubMed
-
- van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. - PubMed
-
- Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

