PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model
- PMID: 19417208
- PMCID: PMC2731636
- DOI: 10.1182/blood-2009-03-206672
PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model
Abstract
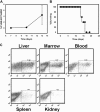
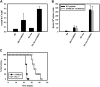
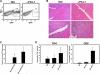
Negative regulatory mechanisms within the solid tumor microenvironment inhibit antitumor T-cell function, leading to evasion from immune attack. One inhibitory mechanism is up-regulation of programmed death-ligand 1 (PD-L1) expressed on tumor or stromal cells which binds to programmed death-1 (PD-1) on activated T cells. PD-1/PD-L1 engagement results in diminished antitumor T-cell responses and correlates with poor outcome in murine and human solid cancers. In contrast to available data in solid tumors, little is known regarding involvement of the PD-1/PD-L1 pathway in immune escape by hematopoietic cancers, such as acute myeloid leukemia (AML). To investigate this hypothesis, we used the murine leukemia, C1498. When transferred intravenously, C1498 cells grew progressively and apparently evaded immune destruction. Low levels of PD-L1 expression were found on C1498 cells grown in vitro. However, PD-L1 expression was up-regulated on C1498 cells when grown in vivo. PD-1(-/-) mice challenged with C1498 cells generated augmented antitumor T-cell responses, showed decreased AML burden in the blood and other organs, and survived significantly longer than did wild-type mice. Similar results were obtained with a PD-L1 blocking antibody. These data suggest the importance of the PD-1/PD-L1 pathway in immune evasion by a hematologic malignancy, providing a rationale for clinical trials targeting this pathway in leukemia patients.
Figures


Comment in
-
Blocking PD-1 in cancer immunotherapy.Blood. 2009 Aug 20;114(8):1457-8. doi: 10.1182/blood-2009-05-223412. Blood. 2009. PMID: 19696208
References
-
- Urban JL, Schreiber H. Tumor antigens. Annu Rev Immunol. 1992;10:617–644. - PubMed
-
- Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. - PubMed
-
- Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. - PubMed
-
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. - PubMed
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

