Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation
- PMID: 19417210
- PMCID: PMC2710944
- DOI: 10.1182/blood-2008-04-151639
Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation
Abstract
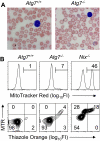
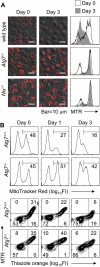
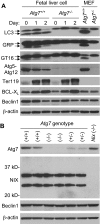
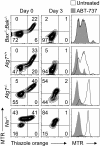
Mitochondrial clearance is a well recognized but poorly understood biologic process, and reticulocytes, which undergo programmed mitochondrial clearance, provide a useful model to study this phenomenon. At the ultrastructural level, mitochondrial clearance resembles an autophagy-related process; however, the role of autophagy in mitochondrial clearance has not been established. Here we provide genetic evidence that autophagy pathways, initially identified in yeast, are involved in mitochondrial clearance from reticulocytes. Atg7 is an autophagy protein and an E1-like enzyme, which is required for the activity of dual ubiquitin-like conjugation pathways. Atg7 is required for the conjugation of Atg12 to Atg5, and Atg8 to phosphatidylethanolamine (PE), and is essential for autophagosome formation. In the absence of Atg7, mitochondrial clearance from reticulocytes is diminished but not completely blocked. Mammalian homologs of Atg8 are unmodified in Atg7(-/-) erythroid cells, indicating that canonical autophagy pathways are inactive. Thus, mitochondrial clearance is regulated by both autophagy-dependent and -independent mechanisms. In addition, mitochondria, which depolarize in wild-type cells before elimination, remain polarized in Atg7(-/-) reticulocytes in culture. This suggests that mitochondrial depolarization is a consequence rather than a cause of autophagosome formation in reticulocytes.
Figures

References
-
- Alexeyev MF, LeDoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci (Lond) 2004;107:355–364. - PubMed
-
- Yen WL, Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 2008;23:248–262. - PubMed
-
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. - PubMed
-
- Gronowicz G, Swift H, Steck TL. Maturation of the reticulocyte in vitro. J Cell Sci. 1984;71:177–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

