Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment
- PMID: 19417580
- PMCID: PMC2739977
- DOI: 10.1097/QAD.0b013e32832b4377
Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment
Abstract
Background: The metabolic effects of initial therapy for HIV-1 infection are important determinants of regimen selection.
Methods: Open-label study in 753 subjects randomized equally to efavirenz or lopinavir/ritonavir(r) plus two nucleoside reverse-transcriptase inhibitor (NRTI) vs. the NRTI-sparing regimen of lopinavir/r plus efavirenz. Zidovudine, stavudine, or tenofovir with lamivudine was selected prior to randomization. Metabolic outcomes through 96 weeks were lipoatrophy, defined as at least 20% loss in extremity fat, and fasting serum lipids.
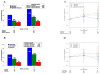
Results: Lipoatrophy by dual-energy X-ray absorptiometry at week 96 occurred in 32% [95% confidence interval (CI) 25-39%] of subjects in the efavirenz plus two NRTIs arm, 17% (95% CI 12-24) in the lopinavir/r plus two NRTIs arm, and 9% (95% CI 5-14) in the NRTI-sparing arm (P < or = 0.023 for all comparisons). Varying the definition of lipoatrophy (> or =10 to > or =40% fat loss) and correction for baseline risk factors did not affect the significant difference in lipoatrophy between the NRTI-containing regimens. Lipoatrophy was most frequent with stavudine-containing regimens and least frequent with tenofovir-containing regimens (P < 0.001), which were not significantly different from the NRTI-sparing regimen. Total cholesterol increases at week 96 were greatest in the NRTI-sparing arm (median +57 mg/dl) compared with the other two arms (+32-33 mg/dl; P < 0.001). Use of lipid-lowering agents was more common (25 vs. 11-13%) in the NRTI-sparing arm.
Conclusion: Lipoatrophy was more frequent with efavirenz than lopinavir/r when combined with stavudine or zidovudine, and less frequent when either drug was combined with tenofovir. Lipoatrophy was least frequent with the NRTI-sparing regimen, but this benefit was offset by greater cholesterol elevations and the need for lipid-lowering agents.
Trial registration: ClinicalTrials.gov NCT00050895.
Conflict of interest statement
Dr. Riddler reports having received lecture or consultation fees from Bristol-Myers Squibb and grant support from Schering-Plough and Hoffman-LaRoche.
Dr. Powderly reports having received lecture or consultation fees from Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Pfizer, Roche, and Tibotec and grant support from GlaxoSmithKline.
Dr. Garren is an employee of Abbott Laboratories.
Dr. George was an employee of Bristol-Myers Squibb.
Dr. Rooney is an employee of Gilead Sciences.
Dr. Haas reports having received research grants from Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Tibotec, Tanox, Gilead Sciences, and Bavarian Nordic, and has served on an Advisory Board for GlaxoSmithKline.
Dr. Mellors reports that he is a consultant to Gilead Sciences, Merck, Panacos, and Idenix Pharmaceuticals, has received grant support from Merck, and owns stock options in RFS Pharma.
Dr. DiRienzo, Ms. Komarow, Dr. Klingman and Dr. Havlir declare that they have no conflicts of interest.
Figures
Comment in
-
Stavudine in antiretroviral therapy: is this the end?AIDS. 2009 Aug 24;23(13):1727-9. doi: 10.1097/QAD.0b013e32832d3c5e. AIDS. 2009. PMID: 19571724 No abstract available.
References
-
- Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. - PubMed
-
- Dube MP, Sprecher D, Henry WK, Aberg JA, Torriani FJ, Hodis HN, et al. Preliminary guidelines for the evaluation and management of dyslipidemia in adults infected with human immunodeficiency virus and receiving antiretroviral therapy: Recommendations of the Adult AIDS Clinical Trial Group Cardiovascular Disease Focus Group. Clin Infect Dis. 2000;31:1216–1224. - PubMed
-
- Wohl DA, McComsey G, Tebas P, Brown TT, Glesby MJ, Reeds D, et al. Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clin Infect Dis. 2006;43:645–653. - PubMed
-
- Carr A, Emery S, Law M, Puls R, Lundgren JD, Powderly WG. An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet. 2003;361:726–735. - PubMed
-
- Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI027661/AI/NIAID NIH HHS/United States
- U01 AI046376/AI/NIAID NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- AI 32783/AI/NIAID NIH HHS/United States
- U01 AI069502/AI/NIAID NIH HHS/United States
- DA 12121/DA/NIDA NIH HHS/United States
- U01 AI069513/AI/NIAID NIH HHS/United States
- AI 069419/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069447/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069513/AI/NIAID NIH HHS/United States
- AI 069465/AI/NIAID NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- AI 069423/AI/NIAID NIH HHS/United States
- AI 060354/AI/NIAID NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- AI 27673/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- AI 069452/AI/NIAID NIH HHS/United States
- AI 069447/AI/NIAID NIH HHS/United States
- AI 32782/AI/NIAID NIH HHS/United States
- RR 02635/RR/NCRR NIH HHS/United States
- RR 00075/RR/NCRR NIH HHS/United States
- U01 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- AI 34853/AI/NIAID NIH HHS/United States
- K24 AI064086/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- AI 069434/AI/NIAID NIH HHS/United States
- RR 00052/RR/NCRR NIH HHS/United States
- AI 46381/AI/NIAID NIH HHS/United States
- AI 069477/AI/NIAID NIH HHS/United States
- UM1 AI069411/AI/NIAID NIH HHS/United States
- AI 068634/AI/NIAID NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- AI 069556/AI/NIAID NIH HHS/United States
- AI 069494/AI/NIAID NIH HHS/United States
- U01 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- AI 36214/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069556/AI/NIAID NIH HHS/United States
- AI 069471/AI/NIAID NIH HHS/United States
- U01 AI069418/AI/NIAID NIH HHS/United States
- AI 069411/AI/NIAID NIH HHS/United States
- M01 RR000039/RR/NCRR NIH HHS/United States
- M01 RR000047/RR/NCRR NIH HHS/United States
- RR 00032/RR/NCRR NIH HHS/United States
- RR 00039/RR/NCRR NIH HHS/United States
- AI 069501/AI/NIAID NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- RR 00046/RR/NCRR NIH HHS/United States
- AI 50410/AI/NIAID NIH HHS/United States
- AI 064086/AI/NIAID NIH HHS/United States
- RR 00051/RR/NCRR NIH HHS/United States
- M01 RR002635/RR/NCRR NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- AI 069472/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI069484/AI/NIAID NIH HHS/United States
- AI 46376/AI/NIAID NIH HHS/United States
- RR 00096/RR/NCRR NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- AI 25859/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- AI 069495/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- U01 AI034853/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- RR 00047/RR/NCRR NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- AI 069484/AI/NIAID NIH HHS/United States
- AI 45008/AI/NIAID NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- AI 069470/AI/NIAID NIH HHS/United States
- UM1 AI069556/AI/NIAID NIH HHS/United States
- AI 069432/AI/NIAID NIH HHS/United States
- U01 AI046381/AI/NIAID NIH HHS/United States
- M01 RR000032/RR/NCRR NIH HHS/United States
- AI 069450/AI/NIAID NIH HHS/United States
- U01 AI027673/AI/NIAID NIH HHS/United States
- AI 27661/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- AI 068636/AI/NIAID NIH HHS/United States
- UM1 AI069502/AI/NIAID NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- U01 AI032782/AI/NIAID NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- AI 069418/AI/NIAID NIH HHS/United States
- U01 AI069411/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- AI 069439/AI/NIAID NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- AI 069424/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- AI 069502/AI/NIAID NIH HHS/United States
- AI 38858/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- AI 069532/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069447/AI/NIAID NIH HHS/United States
- AI 46370/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- U01 AI032783/AI/NIAID NIH HHS/United States
- AI 069513/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- AI 069474/AI/NIAID NIH HHS/United States
- RR 00044/RR/NCRR NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

