Leukocyte adhesion deficiency syndrome: a controversy solved
- PMID: 19417769
- PMCID: PMC4464832
- DOI: 10.1038/icb.2009.32
Leukocyte adhesion deficiency syndrome: a controversy solved
Figures
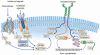
References
-
- Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. (e-pub ahead of print 8 December 2008; doi:10.1182/blood-2008-10-182154) - PubMed
-
- Etzioni A. Leukocyte adhesion deficiencies: molecular basis, clinical findings, and therapeutic options. Adv Exp Med Biol. 2007;601:51–60. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

