Dose-dependent effects of Runx2 on bone development
- PMID: 19419310
- PMCID: PMC2765932
- DOI: 10.1359/jbmr.090502
Dose-dependent effects of Runx2 on bone development
Abstract
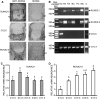
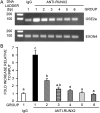
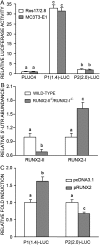
Runx2 controls the commitment of mesenchymal cells to the osteoblastic lineage. Distinct promoters, designated P1 and P2, give rise to functionally similar Runx2-II and Runx2-I isoforms. We postulate that this dual promoter gene structure permits temporal and spatial adjustments in the amount of Runx2 isoforms necessary for optimal bone development. To evaluate the gene dose-dependent effect of Runx2 isoforms on bone development, we intercrossed selective Runx2-II(+/-) with nonselective Runx2-II(+/-)/Runx2-I(+/-) mice to create compound mutant mice: Runx2-II(+/-), Runx2-II(+/-)/Runx2-I(+/-), Runx2-II(-/-), Runx2-II(-/-)/Runx2-I(+/-), Runx2-II(-/-)/Runx2-I(-/-). Analysis of the different Runx2-deficient genotypes showed gene dose-dependent differences in the level of expression of the Runx2 isoforms. In addition, we found that Runx2-I is predominately expressed in the perichondrium and proliferating chondrocytes, whereas Runx2-II is expressed in hypertrophic chondrocytes and metaphyseal osteoblasts. Newborn mice showed impaired development of a mineralized skeleton, bone length, and widening of the hypertrophic zone that were proportionate to the reduction in total Runx2 protein expression. Osteoblast differentiation ex vivo was also proportionate to total amount of Runx2 expression that correlated with reduced Runx2 binding to the osteocalcin promoter by quantitative chromatin immunoprecipitation analysis. Functional analysis of P1 and P2 promoters showed differential regulation of the two promoters in osteoblastic cell lines. These findings support the possibility that the total amount of Runx2 derived from two isoforms and the P1 and P2 promoters, by regulating the time, place, and amount of Runx2 in response to changing environmental cues, impacts on bone development.
Figures




Similar articles
-
Selective deficiency of the "bone-related" Runx2-II unexpectedly preserves osteoblast-mediated skeletogenesis.J Biol Chem. 2004 May 7;279(19):20307-13. doi: 10.1074/jbc.M401109200. Epub 2004 Mar 7. J Biol Chem. 2004. PMID: 15007057
-
Polycystin-1 regulates skeletogenesis through stimulation of the osteoblast-specific transcription factor RUNX2-II.J Biol Chem. 2008 May 2;283(18):12624-34. doi: 10.1074/jbc.M710407200. Epub 2008 Mar 5. J Biol Chem. 2008. PMID: 18321855 Free PMC article.
-
Zfp521 controls bone mass by HDAC3-dependent attenuation of Runx2 activity.J Cell Biol. 2010 Dec 27;191(7):1271-83. doi: 10.1083/jcb.201009107. Epub 2010 Dec 20. J Cell Biol. 2010. PMID: 21173110 Free PMC article.
-
Regulation of bone development and extracellular matrix protein genes by RUNX2.Cell Tissue Res. 2010 Jan;339(1):189-95. doi: 10.1007/s00441-009-0832-8. Epub 2009 Aug 1. Cell Tissue Res. 2010. PMID: 19649655 Review.
-
Roles of Runx2 in Skeletal Development.Adv Exp Med Biol. 2017;962:83-93. doi: 10.1007/978-981-10-3233-2_6. Adv Exp Med Biol. 2017. PMID: 28299652 Review.
Cited by
-
Epigenetic landscape during osteoblastogenesis defines a differentiation-dependent Runx2 promoter region.Gene. 2014 Oct 15;550(1):1-9. doi: 10.1016/j.gene.2014.05.044. Epub 2014 Jun 2. Gene. 2014. PMID: 24881813 Free PMC article.
-
Toward improved phosphorus efficiency in monogastrics-interplay of serum, minerals, bone, and immune system after divergent dietary phosphorus supply in swine.Am J Physiol Regul Integr Comp Physiol. 2016 May 15;310(10):R917-25. doi: 10.1152/ajpregu.00215.2015. Epub 2016 Mar 9. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26962023 Free PMC article. Clinical Trial.
-
Runx2-I is an Early Regulator of Epithelial-Mesenchymal Cell Transition in the Chick Embryo.Dev Dyn. 2018 Mar;247(3):542-554. doi: 10.1002/dvdy.24539. Epub 2017 Jul 19. Dev Dyn. 2018. PMID: 28631378 Free PMC article.
-
Influence of high glucose and advanced glycation end-products (ages) levels in human osteoblast-like cells gene expression.BMC Musculoskelet Disord. 2016 Aug 31;17(1):377. doi: 10.1186/s12891-016-1228-z. BMC Musculoskelet Disord. 2016. PMID: 27582133 Free PMC article.
-
In vitro proliferation and osteogenic differentiation of mesenchymal stem cells on nanoporous alumina.Int J Nanomedicine. 2013;8:2745-56. doi: 10.2147/IJN.S44885. Epub 2013 Jul 29. Int J Nanomedicine. 2013. PMID: 23935364 Free PMC article.
References
-
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. - PubMed
-
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. - PubMed
-
- Choi JY, Pratap J, Javed A, Zaidi SK, Xing L, Balint E, Dalamangas S, Boyce B, van Wijnen AJ, Lian JB, Stein JL, Jones SN, Stein GS. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci USA. 2001;98:8650–8655. - PMC - PubMed
-
- Vaes BL, Ducy P, Sijbers AM, Hendriks JM, van Someren EP, de Jong NG, van den Heuvel ER, Olijve W, van Zoelen EJ, Dechering KJ. Microarray analysis on Runx2-deficient mouse embryos reveals novel Runx2 functions and target genes during intramembranous and endochondral bone formation. Bone. 2006;39:724–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

