Calcium signaling in neurodegeneration
- PMID: 19419557
- PMCID: PMC2689218
- DOI: 10.1186/1750-1326-4-20
Calcium signaling in neurodegeneration
Abstract
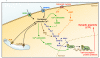
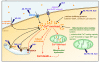
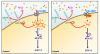
Calcium is a key signaling ion involved in many different intracellular and extracellular processes ranging from synaptic activity to cell-cell communication and adhesion. The exact definition at the molecular level of the versatility of this ion has made overwhelming progress in the past several years and has been extensively reviewed. In the brain, calcium is fundamental in the control of synaptic activity and memory formation, a process that leads to the activation of specific calcium-dependent signal transduction pathways and implicates key protein effectors, such as CaMKs, MAPK/ERKs, and CREB. Properly controlled homeostasis of calcium signaling not only supports normal brain physiology but also maintains neuronal integrity and long-term cell survival. Emerging knowledge indicates that calcium homeostasis is not only critical for cell physiology and health, but also, when deregulated, can lead to neurodegeneration via complex and diverse mechanisms involved in selective neuronal impairments and death. The identification of several modulators of calcium homeostasis, such as presenilins and CALHM1, as potential factors involved in the pathogenesis of Alzheimer's disease, provides strong support for a role of calcium in neurodegeneration. These observations represent an important step towards understanding the molecular mechanisms of calcium signaling disturbances observed in different brain diseases such as Alzheimer's, Parkinson's, and Huntington's diseases.
Figures
Similar articles
-
Neuronal calcium signaling and Alzheimer's disease.Adv Exp Med Biol. 2012;740:1193-217. doi: 10.1007/978-94-007-2888-2_54. Adv Exp Med Biol. 2012. PMID: 22453989 Review.
-
Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer's disease.Ann N Y Acad Sci. 1994 Dec 15;747:77-91. doi: 10.1111/j.1749-6632.1994.tb44402.x. Ann N Y Acad Sci. 1994. PMID: 7847693 Review.
-
Calpain signaling: from biology to therapeutic opportunities in neurodegenerative disorders.Front Vet Sci. 2023 Sep 5;10:1235163. doi: 10.3389/fvets.2023.1235163. eCollection 2023. Front Vet Sci. 2023. PMID: 37732142 Free PMC article. Review.
-
CALHM1 and its polymorphism P86L differentially control Ca²⁺homeostasis, mitogen-activated protein kinase signaling, and cell vulnerability upon exposure to amyloid β.Aging Cell. 2015 Dec;14(6):1094-102. doi: 10.1111/acel.12403. Epub 2015 Sep 29. Aging Cell. 2015. PMID: 26416646 Free PMC article.
-
Synapse-to-Nucleus Signaling in Neurodegenerative and Neuropsychiatric Disorders.Biol Psychiatry. 2019 Jul 15;86(2):87-96. doi: 10.1016/j.biopsych.2019.01.006. Epub 2019 Jan 17. Biol Psychiatry. 2019. PMID: 30846302 Review.
Cited by
-
TRP Channels Role in Pain Associated With Neurodegenerative Diseases.Front Neurosci. 2020 Aug 4;14:782. doi: 10.3389/fnins.2020.00782. eCollection 2020. Front Neurosci. 2020. PMID: 32848557 Free PMC article. Review.
-
Jujuboside A prevents sleep loss-induced disturbance of hippocampal neuronal excitability and memory impairment in young APP/PS1 mice.Sci Rep. 2019 Mar 14;9(1):4512. doi: 10.1038/s41598-019-41114-3. Sci Rep. 2019. PMID: 30872728 Free PMC article.
-
The role of calcium channel blockers and resveratrol in the prevention of paraquat-induced parkinsonism in Drosophila melanogaster: a locomotor analysis.Invert Neurosci. 2011 Jun;11(1):43-51. doi: 10.1007/s10158-011-0116-3. Epub 2011 Apr 27. Invert Neurosci. 2011. PMID: 21523449
-
Transglutaminase 6 Is Colocalized and Interacts with Mutant Huntingtin in Huntington Disease Rodent Animal Models.Int J Mol Sci. 2021 Aug 18;22(16):8914. doi: 10.3390/ijms22168914. Int J Mol Sci. 2021. PMID: 34445621 Free PMC article.
-
The role of Ca2+ signaling in Parkinson's disease.Dis Model Mech. 2017 May 1;10(5):519-535. doi: 10.1242/dmm.028738. Dis Model Mech. 2017. PMID: 28468938 Free PMC article. Review.
References
-
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. - PubMed
-
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. - PubMed
-
- Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: practical and functional considerations. Neuron. 2008;59:902–913. - PubMed
-
- Mattson MP. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988;472:179–212. - PubMed
-
- Redmond L, Ghosh A. Regulation of dendritic development by calcium signaling. Cell Calcium. 2005;37:411–416. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

