Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion
- PMID: 19419954
- PMCID: PMC2709372
- DOI: 10.1074/jbc.M109.006676
Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion
Abstract
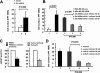
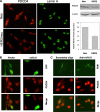
The cell surface receptor tyrosine kinase HER2/neu enhances tumor metastasis. Recent studies suggest that deregulated microRNA (miRNA) expression promotes invasion and metastasis of cancer cells; we therefore explored the possibility that HER2/neu signaling induces the expression of specific miRNAs involved in this process. We identified a putative oncogenic miRNA, miR-21, whose expression is correlated with HER2/neu up-regulation and is functionally involved in HER2/neu-induced cell invasion. We show that miR-21 is up-regulated via the MAPK (ERK1/2) pathway upon stimulation of HER2/neu signaling in breast cancer cells, and overexpression of other ERK1/2 activators such as RASV12 or ID-1 is sufficient to induce miR-21 up-regulation in HER2/neu-negative breast cancer cells. Furthermore, the metastasis suppressor protein PDCD4 (programmed cell death 4) is down-regulated by miR-21 in breast cancer cells expressing HER2/neu. Our data reveal a mechanism for HER2/neu-induced cancer cell invasion via miRNA deregulation. In addition, our results identify miR-21 as a potential therapeutic target for the prevention of breast cancer invasion and metastasis.
Figures




References
-
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A., et al. ( 1989) Science 244, 707– 712 - PubMed
-
- Yu D., Hung M. C. ( 2000) Oncogene 19, 6115– 6121 - PubMed
-
- Tan M., Yao J., Yu D. ( 1997) Cancer Res. 57, 1199– 1205 - PubMed
-
- Subbaramaiah K., Norton L., Gerald W., Dannenberg A. J. ( 2002) J. Biol. Chem. 277, 18649– 18657 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

