Substrate binding tunes conformational flexibility and kinetic stability of an amino acid antiporter
- PMID: 19419962
- PMCID: PMC2707244
- DOI: 10.1074/jbc.M109.004267
Substrate binding tunes conformational flexibility and kinetic stability of an amino acid antiporter
Abstract
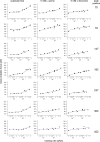
We used single molecule dynamic force spectroscopy to unfold individual serine/threonine antiporters SteT from Bacillus subtilis. The unfolding force patterns revealed interactions and energy barriers that stabilized structural segments of SteT. Substrate binding did not establish strong localized interactions but appeared to be facilitated by the formation of weak interactions with several structural segments. Upon substrate binding, all energy barriers of the antiporter changed thereby describing the transition from brittle mechanical properties of SteT in the unbound state to structurally flexible conformations in the substrate-bound state. The lifetime of the unbound state was much shorter than that of the substrate-bound state. This leads to the conclusion that the unbound state of SteT shows a reduced conformational flexibility to facilitate specific substrate binding and a reduced kinetic stability to enable rapid switching to the bound state. In contrast, the bound state of SteT showed an increased conformational flexibility and kinetic stability such as required to enable transport of substrate across the cell membrane. This result supports the working model of antiporters in which alternate substrate access from one to the other membrane surface occurs in the substrate-bound state.
Figures




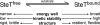
Similar articles
-
Role of transmembrane domain 8 in substrate selectivity and translocation of SteT, a member of the L-amino acid transporter (LAT) family.J Biol Chem. 2010 Sep 10;285(37):28764-76. doi: 10.1074/jbc.M110.116632. Epub 2010 Jul 7. J Biol Chem. 2010. PMID: 20610400 Free PMC article.
-
Crystal structure of Ca2+/H+ antiporter protein YfkE reveals the mechanisms of Ca2+ efflux and its pH regulation.Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):11367-72. doi: 10.1073/pnas.1302515110. Epub 2013 Jun 24. Proc Natl Acad Sci U S A. 2013. PMID: 23798403 Free PMC article.
-
Substrate recognition and transport behavior analyses of amino acid antiporter with coarse-grained models.Mol Biosyst. 2010 Dec;6(12):2430-8. doi: 10.1039/c005266c. Epub 2010 Sep 14. Mol Biosyst. 2010. PMID: 20838682
-
Tet(L) and tet(K) tetracycline-divalent metal/H+ antiporters: characterization of multiple catalytic modes and a mutagenesis approach to differences in their efflux substrate and coupling ion preferences.J Bacteriol. 2002 Sep;184(17):4722-32. doi: 10.1128/JB.184.17.4722-4732.2002. J Bacteriol. 2002. PMID: 12169596 Free PMC article.
-
Metamorphosis of research on ion-coupled metabolite transport.J Exp Biol. 1994 Nov;196:139-44. doi: 10.1242/jeb.196.1.139. J Exp Biol. 1994. PMID: 7823017 Review. No abstract available.
Cited by
-
Substrate-binding guides individual melibiose permeases MelB to structurally soften and to destabilize cytoplasmic middle-loop C3.Structure. 2023 Jan 5;31(1):58-67.e4. doi: 10.1016/j.str.2022.11.011. Epub 2022 Dec 15. Structure. 2023. PMID: 36525976 Free PMC article.
-
Conservation of molecular interactions stabilizing bovine and mouse rhodopsin.Biochemistry. 2010 Dec 14;49(49):10412-20. doi: 10.1021/bi101345x. Epub 2010 Nov 11. Biochemistry. 2010. PMID: 21038881 Free PMC article.
-
Competing interactions stabilize pro- and anti-aggregant conformations of human Tau.J Biol Chem. 2011 Jun 10;286(23):20512-24. doi: 10.1074/jbc.M111.237875. Epub 2011 Apr 15. J Biol Chem. 2011. PMID: 21498513 Free PMC article.
-
Integrated AlphaFold2 and DEER investigation of the conformational dynamics of a pH-dependent APC antiporter.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2206129119. doi: 10.1073/pnas.2206129119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969794 Free PMC article.
-
Locating an extracellular K+-dependent interaction site that modulates betaine-binding of the Na+-coupled betaine symporter BetP.Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):E890-8. doi: 10.1073/pnas.1109597108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987793 Free PMC article.
References
-
- Jack D. L., Paulsen I. T., Saier M. H., Jr. (2000) Microbiology 146, 1797–1814 - PubMed
-
- Verrey F., Jack D. L., Paulsen I. T., Saier M. H., Jr., Pfeiffer R. (1999) J. Membr. Biol. 172, 181–192 - PubMed
-
- Palacín M., Nunes V., Font-Llitjós M., Jiménez-Vidal M., Fort J., Gasol E., Pineda M., Feliubadaló L., Chillarón J., Zorzano A. (2005) Physiology 20, 112–124 - PubMed
-
- Calonge M. J., Gasparini P., Chillarón J., Chillón M., Gallucci M., Rousaud F., Zelante L., Testar X., Dallapiccola B., Di Silverio F., Barceló P., Estivill X., Zorzano A., Nunes V., Palacín M. (1994) Nat. Genet. 6, 420–425 - PubMed
-
- Feliubadaló L., Font M., Purroy J., Rousaud F., Estivill X., Nunes V., Golomb E., Centola M., Aksentijevich I., Kreiss Y., Goldman B., Pras M., Kastner D. L., Pras E., Gasparini P., Bisceglia L., Beccia E., Gallucci M., de Sanctis L., Ponzone A., Rizzoni G. F., Zelante L., Bassi M. T., George A. L., Jr., Manzoni M., De Grandi A., Riboni M., Endsley J. K., Ballabio A., Borsani G., Reig N., Fernández E., Estévez R., Pineda M., Torrents D., Camps M., Lloberas J., Zorzano A., Palacín M. (1999) Nat. Genet. 23, 52–57 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

