Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues
- PMID: 19420011
- PMCID: PMC2710976
- DOI: 10.1677/JOE-09-0066
Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues
Abstract
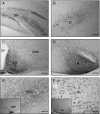
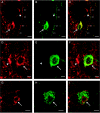
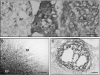
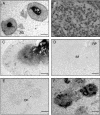
Recently, the G protein-coupled receptor GPR30 has been identified as a novel oestrogen receptor (ER). The distribution of the receptor has been thus far mapped only in the rat central nervous system. This study was undertaken to map the distribution of GPR30 in the mouse brain and rodent peripheral tissues. Immunohistochemistry using an antibody against GPR30 revealed high levels of GPR30 immunoreactivity (ir) in the forebrain (e.g. cortex, hypothalamus and hippocampus), specific nuclei of the midbrain (e.g. the pontine nuclei and locus coeruleus) and the trigeminal nuclei and cerebellum Purkinje layer of the hindbrain in the adult mouse brain. In the rat and mouse periphery, GPR30-ir was detected in the anterior, intermediate and neural lobe of the pituitary, adrenal medulla, renal pelvis and ovary. In situ hybridisation histochemistry using GPR30 riboprobes, revealed intense hybridisation signal for GPR30 in the paraventricular nucleus and supraoptic nucleus (SON) of the hypothalamus, anterior and intermediate lobe of the pituitary, adrenal medulla, renal pelvis and ovary of both rat and mouse. Double immunofluorescence revealed GPR30 was present in both oxytocin and vasopressin neurones of the paraventricular nucleus and SON of the rat and mouse brain. The distribution of GPR30 is distinct from the other traditional ERs and offers an additional way in which oestrogen may mediate its effects in numerous brain regions and endocrine systems in the rodent.
Figures




Similar articles
-
Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei.Endocrinology. 2007 Dec;148(12):5842-50. doi: 10.1210/en.2007-0436. Epub 2007 Sep 13. Endocrinology. 2007. PMID: 17872373
-
Central and peripheral apelin receptor distribution in the mouse: species differences with rat.Peptides. 2012 Jan;33(1):139-48. doi: 10.1016/j.peptides.2011.12.005. Epub 2011 Dec 16. Peptides. 2012. PMID: 22197493 Free PMC article.
-
Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta.Prog Brain Res. 2002;139:15-29. doi: 10.1016/s0079-6123(02)39004-6. Prog Brain Res. 2002. PMID: 12436923 Review.
-
Novel G protein-coupled oestrogen receptor GPR30 shows changes in mRNA expression in the rat brain over the oestrous cycle.Neurosignals. 2013;21(1-2):14-27. doi: 10.1159/000333296. Epub 2012 Feb 23. Neurosignals. 2013. PMID: 22378360
-
In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo.Endocr J. 2010;57(2):101-7. doi: 10.1507/endocrj.k09e-332. Epub 2009 Dec 8. Endocr J. 2010. PMID: 19996532 Review.
Cited by
-
Estrogen promotes luteolysis by redistributing prostaglandin F2α receptors within primate luteal cells.Reproduction. 2015 May;149(5):453-64. doi: 10.1530/REP-14-0412. Epub 2015 Feb 16. Reproduction. 2015. PMID: 25687410 Free PMC article.
-
Male/Female differences in neuroprotection and neuromodulation of brain dopamine.Front Endocrinol (Lausanne). 2011 Sep 30;2:35. doi: 10.3389/fendo.2011.00035. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654803 Free PMC article.
-
Evaluating the Role of Hormone Therapy in Postmenopausal Women with Alzheimer's Disease.Drugs Aging. 2016 Nov;33(11):787-808. doi: 10.1007/s40266-016-0407-9. Drugs Aging. 2016. PMID: 27709466 Review.
-
Estrogen receptors observed at extranuclear neuronal sites and in glia in the nucleus accumbens core and shell of the female rat: Evidence for localization to catecholaminergic and GABAergic neurons.J Comp Neurol. 2022 Aug;530(11):2056-2072. doi: 10.1002/cne.25320. Epub 2022 Apr 9. J Comp Neurol. 2022. PMID: 35397175 Free PMC article.
-
Estrogens of multiple classes and their role in mental health disease mechanisms.Int J Womens Health. 2010 Aug 9;2:153-66. doi: 10.2147/ijwh.s6907. Int J Womens Health. 2010. PMID: 21072308 Free PMC article.
References
-
- Ábrahám I, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signalling in mouse brain. Endocrinology. 2004;145:3055–3061. - PubMed
-
- Adashi EY. Endocrinology of the ovary. Human Reproduction. 1994;9:815–827. - PubMed
-
- Adsay NV, Eble JN, Srigley JR, Jones EC, Grignon DJ. Mixed epithelial and stromal tumor of the kidney. American Journal of Surgical Pathology. 2000;24:958–970. - PubMed
-
- Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regulatory Peptides. 2005;15:7–17. - PubMed
-
- Baquedano MS, Saraco N, Berensztein E, Pepe C, Bianchini M, Levy E, Goñi J, Rivarola MA, Belgorosky A. Identification and developmental changes of aromatase and estrogen receptor expression in prepubertal and pubertal human adrenal tissues. Journal of Clinical Endocrinology and Metabolism. 2007;92:2215–2222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

