Expression of the RNA-stabilizing protein HuR in ischemia-reperfusion injury of rat kidney
- PMID: 19420108
- PMCID: PMC2711713
- DOI: 10.1152/ajprenal.90632.2008
Expression of the RNA-stabilizing protein HuR in ischemia-reperfusion injury of rat kidney
Abstract
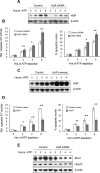
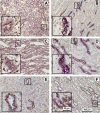
The RNA-binding protein human antigen R (HuR) participates in the posttranscriptional regulation of mRNAs bearing 3' AU-rich and U-rich elements, which HuR can stabilize under conditions of cellular stress. Using the LLC-PK(1) proximal tubule cell line model, we recently suggested a role for HuR in protecting kidney epithelia from injury during ischemic stress (Jeyaraj S, Dakhlallah D, Hill SR, Lee BS. J Biol Chem 280: 37957-37964, 2005; Jeyaraj SC, Dakhlallah D, Hill SR, Lee BS. Am J Physiol Renal Physiol 291: F1255-F1263, 2006). Here, we have extended this work to show that small interfering RNA-mediated suppression of HuR in LLC-PK(1) cells increased apoptosis during energy depletion, while overexpression of HuR diminished apoptosis. Suppression of HuR also resulted in diminished levels of key cell survival proteins such as Bcl-2 and Hsp70. Furthermore, rat kidneys were subjected in vivo to transient ischemia followed by varying periods of reperfusion. Ischemia and reperfusion (I/R) affected intensity and distribution of HuR in a nephron segment-specific manner. Cells of the proximal tubule, which are most sensitive to I/R injury, demonstrated a transient shift of HuR to the cytoplasm immediately following ischemia. Over a 14-day period following the onset of reperfusion, nuclear and total HuR protein gradually increased in cortical and medullary proximal tubules, but not in non-proximal tubule cells. HuR mRNA was expressed in two forms with alternate transcriptional start sites that increased over a 14-day I/R period, and in vitro studies suggest selective translatability of these two mRNAs. Baseline and I/R-stimulated levels of HuR mRNA did not parallel those of HuR protein, suggesting translational control of HuR expression, particularly in medullary proximal tubules. These findings suggest that alterations in distribution and expression of the antiaptotic protein HuR specifically in cells of the proximal tubule effect a protective mechanism during and following I/R injury in kidney.
Figures
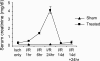





Similar articles
-
Expression and distribution of HuR during ATP depletion and recovery in proximal tubule cells.Am J Physiol Renal Physiol. 2006 Dec;291(6):F1255-63. doi: 10.1152/ajprenal.00440.2005. Epub 2006 Jun 20. Am J Physiol Renal Physiol. 2006. PMID: 16788138 Free PMC article.
-
Transcriptional control of human antigen R by bone morphogenetic protein.J Biol Chem. 2010 Feb 12;285(7):4432-40. doi: 10.1074/jbc.M109.062216. Epub 2009 Dec 14. J Biol Chem. 2010. PMID: 20018854 Free PMC article.
-
Polyamines regulate the stability of activating transcription factor-2 mRNA through RNA-binding protein HuR in intestinal epithelial cells.Mol Biol Cell. 2007 Nov;18(11):4579-90. doi: 10.1091/mbc.e07-07-0675. Epub 2007 Sep 5. Mol Biol Cell. 2007. PMID: 17804813 Free PMC article.
-
HuR and mRNA stability.Cell Mol Life Sci. 2001 Feb;58(2):266-77. doi: 10.1007/PL00000854. Cell Mol Life Sci. 2001. PMID: 11289308 Free PMC article. Review.
-
HuR, a key post-transcriptional regulator, and its implication in progression of breast cancer.Histol Histopathol. 2010 Oct;25(10):1331-40. doi: 10.14670/HH-25.1331. Histol Histopathol. 2010. PMID: 20712017 Review.
Cited by
-
HuR inhibits apoptosis by amplifying Akt signaling through a positive feedback loop.J Cell Physiol. 2013 Jan;228(1):182-9. doi: 10.1002/jcp.24120. J Cell Physiol. 2013. PMID: 22674407 Free PMC article.
-
Integrated bioinformatics and experiments reveal the roles and driving forces for HSF1 in colorectal cancer.Bioengineered. 2022 Feb;13(2):2536-2552. doi: 10.1080/21655979.2021.2018235. Bioengineered. 2022. PMID: 35006040 Free PMC article.
-
Serum-stabilized naked caspase-3 siRNA protects autotransplant kidneys in a porcine model.Mol Ther. 2014 Oct;22(10):1817-28. doi: 10.1038/mt.2014.111. Epub 2014 Jun 16. Mol Ther. 2014. PMID: 24930602 Free PMC article.
-
Preconditioning strategies for kidney ischemia reperfusion injury: implications of the "time-window" in remote ischemic preconditioning.PLoS One. 2015 Apr 16;10(4):e0124130. doi: 10.1371/journal.pone.0124130. eCollection 2015. PLoS One. 2015. PMID: 25879855 Free PMC article.
-
Multifaceted Human Antigen R (HuR): A Key Player in Liver Metabolism and MASLD.Livers. 2025 Sep;5(3):33. doi: 10.3390/livers5030033. Epub 2025 Jul 21. Livers. 2025. PMID: 40809813 Free PMC article.
References
-
- Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle 6: 1288–1292, 2007. - PubMed
-
- Agre P Homer W. Smith award lecture. Aquaporin water channels in kidney. J Am Soc Nephrol 11: 764–777, 2000. - PubMed
-
- Amadio M, Scapagnini G, Laforenza U, Intrieri M, Romeo L, Govoni S, Pascale A. Post-transcriptional regulation of HSP70 expression following oxidative stress in SH-SY5Y cells: the potential involvement of the RNA-binding protein HuR. Curr Pharm Des 14: 2651–2658, 2008. - PubMed
-
- Aufricht C Heat-shock protein 70: molecular supertool? Pediatr Nephrol 20: 707–713, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

