IC138 defines a subdomain at the base of the I1 dynein that regulates microtubule sliding and flagellar motility
- PMID: 19420135
- PMCID: PMC2704157
- DOI: 10.1091/mbc.e09-04-0277
IC138 defines a subdomain at the base of the I1 dynein that regulates microtubule sliding and flagellar motility
Abstract
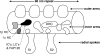
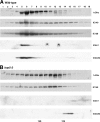
To understand the mechanisms that regulate the assembly and activity of flagellar dyneins, we focused on the I1 inner arm dynein (dynein f) and a null allele, bop5-2, defective in the gene encoding the IC138 phosphoprotein subunit. I1 dynein assembles in bop5-2 axonemes but lacks at least four subunits: IC138, IC97, LC7b, and flagellar-associated protein (FAP) 120--defining a new I1 subcomplex. Electron microscopy and image averaging revealed a defect at the base of the I1 dynein, in between radial spoke 1 and the outer dynein arms. Microtubule sliding velocities also are reduced. Transformation with wild-type IC138 restores assembly of the IC138 subcomplex and rescues microtubule sliding. These observations suggest that the IC138 subcomplex is required to coordinate I1 motor activity. To further test this hypothesis, we analyzed microtubule sliding in radial spoke and double mutant strains. The results reveal an essential role for the IC138 subcomplex in the regulation of I1 activity by the radial spoke/phosphorylation pathway.
Figures
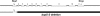


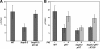
References
-
- Brokaw C. J. Control of flagellar bending: a new agenda based on dynein diversity. Cell Motil. Cytoskeleton. 1994;28:199–204. - PubMed
-
- Brokaw C. J. Thinking about flagellar oscillation. Cell Motil. Cytoskeleton. 2008 in press. - PubMed
-
- Brokaw C. J., Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskeleton. 1987;8:68–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

