Balance point characterization of interstitial fluid volume regulation
- PMID: 19420292
- PMCID: PMC2711695
- DOI: 10.1152/ajpregu.00097.2009
Balance point characterization of interstitial fluid volume regulation
Abstract
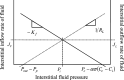
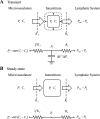
The individual processes involved in interstitial fluid volume and protein regulation (microvascular filtration, lymphatic return, and interstitial storage) are relatively simple, yet their interaction is exceedingly complex. There is a notable lack of a first-order, algebraic formula that relates interstitial fluid pressure and protein to critical parameters commonly used to characterize the movement of interstitial fluid and protein. Therefore, the purpose of the present study is to develop a simple, transparent, and general algebraic approach that predicts interstitial fluid pressure (P(i)) and protein concentrations (C(i)) that takes into consideration all three processes. Eight standard equations characterizing fluid and protein flux were solved simultaneously to yield algebraic equations for P(i) and C(i) as functions of parameters characterizing microvascular, interstitial, and lymphatic function. Equilibrium values of P(i) and C(i) arise as balance points from the graphical intersection of transmicrovascular and lymph flows (analogous to Guyton's classical cardiac output-venous return curves). This approach goes beyond describing interstitial fluid balance in terms of conservation of mass by introducing the concept of inflow and outflow resistances. Algebraic solutions demonstrate that P(i) and C(i) result from a ratio of the microvascular filtration coefficient (1/inflow resistance) and effective lymphatic resistance (outflow resistance), and P(i) is unaffected by interstitial compliance. These simple algebraic solutions predict P(i) and C(i) that are consistent with reported measurements. The present work therefore presents a simple, transparent, and general balance point characterization of interstitial fluid balance resulting from the interaction of microvascular, interstitial, and lymphatic function.
Figures




References
-
- Arturson G, Mellander S. Acute changes in capillary filtration and diffusion in experimental burn injury. Acta Physiol Scand 62: 457–463, 1964. - PubMed
-
- Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73: 1–78, 1993. - PubMed
-
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989. - PubMed
-
- Bentzer P, Kongstad L, Grande PO. Capillary filtration coefficient is independent of number of perfused capillaries in cat skeletal muscle. Am J Physiol Heart Circ Physiol 280: H2697–H2706, 2001. - PubMed
-
- Bert JL, Gyenge CC, Bowen BD, Reed RK, Lund T. A model of fluid and solute exchange in the human: validation and implications. Acta Physiol Scand 170: 201–209, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

