Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients
- PMID: 19421167
- PMCID: PMC2991048
- DOI: 10.1038/tpj.2009.14
Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients
Abstract
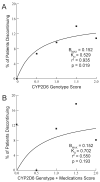
The selective estrogen receptor modulator tamoxifen is routinely used for treatment and prevention of estrogen-receptor-positive breast cancer. Studies of tamoxifen adherence suggest that over half of patients discontinue treatment before the recommended 5 years. We hypothesized that polymorphisms in CYP2D6, the enzyme responsible for tamoxifen activation, predict for tamoxifen discontinuation. Tamoxifen-treated women (n=297) were genotyped for CYP2D6 variants and assigned a 'score' based on predicted allele activities from 0 (no activity) to 2 (high activity). Correlation between CYP2D6 score and discontinuation rates at 4 months was tested. We observed a strong nonlinear correlation between higher CYP2D6 score and increased rates of discontinuation (r(2)=0.935, P=0.018). These data suggest that presence of active CYP2D6 alleles may predict for higher likelihood of tamoxifen discontinuation. Therefore, patients who may be most likely to benefit from tamoxifen may paradoxically be most likely to discontinue treatment prematurely.
Conflict of interest statement
JMR has received research funding Pfizer and speaking honoraria for Roche Diagnostics.
TS has received speaking honoraria for Roche Diagnostics.
DAF is on the Scientific Advisory Board of Labcorp, Inc, is a consultant to Roche Molecular Diagnostics, and has received research funding from Pfizer and Novartis.
DFH has received research funding from AstraZeneca, Glaxo-Smith Kline, Pfizer, and Novartis.
VS has served as a consultant to Wyeth Pharmaceuticals, Concert Pharmaceuticals and JDS Pharmaceuticals, and has received research funding from Glaxo-Smith Kline, Pfizer, and Novartis.
Figures

References
-
- Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. - PubMed
-
- Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. - PubMed
-
- Stewart HJ, Prescott RJ, Forrest AP. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst. 2001;93:456–462. - PubMed
-
- Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

