Safety and pharmacokinetic trial of docetaxel plus an Astragalus-based herbal formula for non-small cell lung cancer patients
- PMID: 19421753
- PMCID: PMC3746541
- DOI: 10.1007/s00280-009-1003-z
Safety and pharmacokinetic trial of docetaxel plus an Astragalus-based herbal formula for non-small cell lung cancer patients
Abstract
Purpose: To study a commonly used Astragalus-based herbal formula previously found effective in non-small cell lung cancer (NSCLC) on the pharmacokinetics of docetaxel in patients with NSCLC.
Methods: Patients with advanced NSCLC who progressed after prior platinum-containing chemotherapy were accrued and received docetaxel at 35 mg/m(2) for 3 weeks followed by 1 week of rest. At 4 days prior to the second dosing, Jinfukang was given orally. Pharmacokinetic studies of initial-dose docetaxel (in the absence of Jinfukang) and the third dose (in the presence of Jinfukang) were compared.
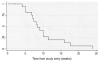
Results: Of the 24 patients enrolled, 21 started Jinfukang and docetaxel. Jinfukang had no significant impact on the pharmacokinetics of docetaxel. Median time to progression or withdrawal from treatment was 7 weeks. Twelve patients were removed from study for progression of disease; nine patients withdrew.
Conclusions: Jinfukang did not alter the pharmacokinetics of docetaxel nor appear to affect survival in this study.
Figures
Similar articles
-
Phase I/II study of docetaxel combined with resminostat, an oral hydroxamic acid HDAC inhibitor, for advanced non-small cell lung cancer in patients previously treated with platinum-based chemotherapy.Invest New Drugs. 2017 Apr;35(2):217-226. doi: 10.1007/s10637-017-0435-2. Epub 2017 Jan 30. Invest New Drugs. 2017. PMID: 28138828 Clinical Trial.
-
Phase I dose-finding and pharmacokinetic study of docetaxel and gefitinib in patients with advanced or metastatic non-small-cell lung cancer: evaluation of drug-drug interaction.Cancer Chemother Pharmacol. 2015 Oct;76(4):713-21. doi: 10.1007/s00280-015-2837-1. Epub 2015 Aug 2. Cancer Chemother Pharmacol. 2015. PMID: 26233803 Clinical Trial.
-
A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy.Lung Cancer. 2016 Sep;99:186-93. doi: 10.1016/j.lungcan.2016.07.019. Epub 2016 Jul 18. Lung Cancer. 2016. PMID: 27565938 Clinical Trial.
-
Bortezomib plus docetaxel in advanced non-small cell lung cancer and other solid tumors: a phase I California Cancer Consortium trial.J Thorac Oncol. 2006 Feb;1(2):126-34. J Thorac Oncol. 2006. PMID: 17409841 Clinical Trial.
-
Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the Western dose.Cancer Sci. 2015 May;106(5):497-504. doi: 10.1111/cas.12647. Epub 2015 Mar 25. Cancer Sci. 2015. PMID: 25728850 Free PMC article. Review.
Cited by
-
Natural persistence of the coastal plant Glehnia littoralis along temperate sandy coasts.Sci Rep. 2017 Feb 17;7:42784. doi: 10.1038/srep42784. Sci Rep. 2017. PMID: 28211487 Free PMC article.
-
Herbal formula Yangyinjiedu induces lung cancer cell apoptosis via activation of early growth response 1.J Cell Mol Med. 2019 Sep;23(9):6193-6202. doi: 10.1111/jcmm.14501. Epub 2019 Jun 25. J Cell Mol Med. 2019. PMID: 31237749 Free PMC article.
-
Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study.J Evid Based Complementary Altern Med. 2017 Oct;22(4):982-995. doi: 10.1177/2156587217696927. Epub 2017 Mar 31. J Evid Based Complementary Altern Med. 2017. PMID: 28359161 Free PMC article. Review.
-
Herbal formula YYJD inhibits tumor growth by inducing cell cycle arrest and senescence in lung cancer.Sci Rep. 2017 Jul 10;7(1):4984. doi: 10.1038/s41598-017-05146-x. Sci Rep. 2017. PMID: 28694520 Free PMC article.
-
Establishment of a Nomogram-Based Prognostic Model (LASSO-COX Regression) for Predicting Progression-Free Survival of Primary Non-Small Cell Lung Cancer Patients Treated with Adjuvant Chinese Herbal Medicines Therapy: A Retrospective Study of Case Series.Front Oncol. 2022 Jul 8;12:882278. doi: 10.3389/fonc.2022.882278. eCollection 2022. Front Oncol. 2022. PMID: 35875082 Free PMC article.
References
-
- Socinski MA, Morris DE, Masters GA, Lilenbaum R. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. 2003;123:226S–243S. - PubMed
-
- Liu J, Shi Z, Li H, Xu Z, Zhu Y, Zhao L, et al. Clinical observation on 271 cases of non-small lung cancer treated with Yifei Kangliu Yin (Jin Fu Kang) Chin J Integr Tradit West Med. 2001;7:247–250.
-
- McCulloch M, See C, Shu XJ, Broffman M, Kramer A, Fan WY, Gao J, Lieb W, Shieh K, Colford JM., Jr Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small cell lung cancer: meta-analysis of randomized trials. J Clin Oncol. 2006;24:419–430. - PubMed
-
- Liu JPM, Li Y, Ye D, Guo Y, Li Y. Clinical study of oral liquid Jin Fu Kang for the treatment of primary non-small cell lung cancer. Tumor (Shanghai) 2001;21:463–465.
-
- Madabushi R, Frank B, Drewelow B, Derendorf H, Butterweck V. Hyperforin in St. John’s wort drug interactions. Eur J Clin Pharmacol. 2006;62:225–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical