Transcription profiling of HCN-channel isotypes throughout mouse cardiac development
- PMID: 19421833
- PMCID: PMC2758203
- DOI: 10.1007/s00395-009-0031-5
Transcription profiling of HCN-channel isotypes throughout mouse cardiac development
Abstract
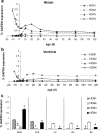
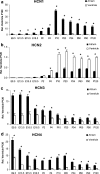
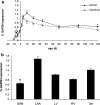
Hyperpolarization-activated ion channels, encoded by four mammalian genes (HCN1-4), contribute in an important way to the cardiac pacemaker current I(f). Here, we describe the transcription profiles of the four HCN genes, the NRSF, KCNE2 and Kir2.1 genes from embryonic stage E9.5 dpc to postnatal day 120 in the mouse. Embryonic atrium and ventricle revealed abundant HCN4 transcription but other HCN transcripts were almost absent. Towards birth, HCN4 was downregulated in the atrium and almost vanished from the ventricle. After birth, however, HCN isotype transcription changed remarkably, showing increased levels of HCN1, HCN2 and HCN4 in the atrium and of HCN2 and HCN4 in the ventricle. HCN3 showed highest transcription at early embryonic stages and was hardly detectable thereafter. At postnatal day 10, HCN4 was highest in the sinoatrial node, being twofold higher than HCN1 and fivefold higher than HCN2. In the atrium, HCN4 was similar to HCN1 and sevenfold higher than HCN2. In the ventricle, in contrast, HCN2 was sixfold higher than HCN4, while HCN1 was absent. Subsequently all HCN isotype transcripts declined to lower adult levels, while ratios of HCN isotypes remained stable. In conclusion, substantial changes of HCN isotype transcription throughout cardiac development suggest that a regulated pattern of HCN isotypes is required to establish and ensure a stable heart rhythm. Furthermore, constantly low HCN transcription in adult myocardium may be required to prevent atrial and ventricular arrhythmogenesis.
Figures


References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1113/jphysiol.2002.027698', 'is_inner': False, 'url': 'https://doi.org/10.1113/jphysiol.2002.027698'}, {'type': 'PMC', 'value': 'PMC2342966', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2342966/'}, {'type': 'PubMed', 'value': '12702747', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12702747/'}]}
- Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, Moroni A, Baruscotti M, DiFrancesco D (2003) Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol 549:347–359 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1096/fj.02-0889com', 'is_inner': False, 'url': 'https://doi.org/10.1096/fj.02-0889com'}, {'type': 'PubMed', 'value': '12958166', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12958166/'}]}
- Borlak J, Thum T (2003) Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J 17:1592–1608 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15365256', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15365256/'}]}
- Chun KRJ, Koenen M, Katus HA, Zehelein J (2004) Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development. Exp Mol Med 36:367–371 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1161/CIRCULATIONAHA.106.616011', 'is_inner': False, 'url': 'https://doi.org/10.1161/circulationaha.106.616011'}, {'type': 'PubMed', 'value': '17420362', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17420362/'}]}
- Dobrzynski H, Boyett MR, Anderson RH (2007) New insights into pacemaker activity promoting understanding of sick sinus syndrome. Circulation 115:1921–1932 - PubMed
-
- None
- DiFrancesco D (1996) The hyperpolarization-activated (If) current: autonomic regulation and the control of pacing. In: Morad M, Ebashi S, Trautwein W, Kurachi Y (eds) Molecular physiology and pharmacology of cardiac ion channels and transporters. Kluwer Academic Publishers, Dordrecht, pp 31–37
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

