Characterization of P2Y receptor subtypes functionally expressed on neonatal rat cardiac myofibroblasts
- PMID: 19422377
- PMCID: PMC2795244
- DOI: 10.1111/j.1476-5381.2009.00172.x
Characterization of P2Y receptor subtypes functionally expressed on neonatal rat cardiac myofibroblasts
Abstract
Background and purpose: Little is known about P2Y receptors in cardiac fibroblasts, which represent the predominant cell type in the heart and differentiate into myofibroblasts under certain conditions. Therefore, we have characterized the phenotype of the cells and the different P2Y receptors at the expression and functional levels in neonatal rat non-cardiomyocytes.
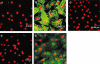
Experimental approach: Non-cardiomyocyte phenotype was determined by confocal microscopy by using discoidin domain receptor 2, alpha-actin and desmin antibodies. P2Y receptor expression was investigated by reverse transcription-polymerase chain reaction and immunocytochemistry, and receptor function by cAMP and inositol phosphate (IP) accumulation induced by adenine or uracil nucleotides in the presence or absence of selective antagonists of P2Y(1) (MRS 2179, 2-deoxy-N(6)-methyl adenosine 3',5'-diphosphate diammonium salt), P2Y(6) (MRS 2578) and P2Y(11) (NF 157, 8,8'-[carbonylbis[imino-3,1-phenylenecarbonylimino(4-fluoro-3,1-phenylene)carbonylimino]]bis-1,3,5-naphthalene trisulphonic acid hexasodium salt) receptors. G(i/o) and G(q/11) pathways were evaluated by using Pertussis toxin and YM-254890 respectively.
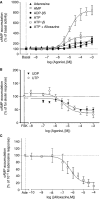
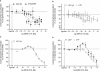
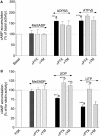
Key results: The cells (>95%) were alpha-actin and discoidin domain receptor 2-positive and desmin-negative. P2Y(1), P2Y(2), P2Y(4), P2Y(6) were detected by reverse transcription-polymerase chain reaction and immunocytochemistry, and P2Y(11)-like receptors at protein level. All di- or tri-phosphate nucleotides stimulated IP production in an YM-254890-sensitive manner. AMP, ADPbetaS, ATP and ATPgammaS increased cAMP accumulation, whereas UDP and UTP inhibited cAMP response, which was abolished by Pertussis toxin. MRS 2179 and NF 157 inhibited ADPbetaS-induced IP production. MRS 2578 blocked UDP- and UTP-mediated IP responses.
Conclusion and implications: P2Y(1)-, P2Y(2)-, P2Y(4)-, P2Y(6)-, P2Y(11)-like receptors were co-expressed and induced function through G(q/11) protein coupling in myofibroblasts. Furthermore, P2Y(2) and P2Y(4) receptor subtypes were also coupled to G(i/o). The G(s) response to adenine nucleotides suggests a possible expression of a new P2Y receptor subtype.
Figures




References
-
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodelling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

