Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates
- PMID: 19422423
- PMCID: PMC2736117
- DOI: 10.1111/j.1469-7580.2009.01046.x
Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates
Abstract
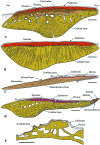
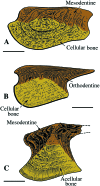
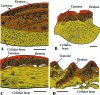
Most non-tetrapod vertebrates develop mineralized extra-oral elements within the integument. Known collectively as the integumentary skeleton, these elements represent the structurally diverse skin-bound contribution to the dermal skeleton. In this review we begin by summarizing what is known about the histological diversity of the four main groups of integumentary skeletal tissues: hypermineralized (capping) tissues; dentine; plywood-like tissues; and bone. For most modern taxa, the integumentary skeleton has undergone widespread reduction and modification often rendering the homology and relationships of these elements confused and uncertain. Fundamentally, however, all integumentary skeletal elements are derived (alone or in combination) from only two types of cell condensations: odontogenic and osteogenic condensations. We review the origin and diversification of the integumentary skeleton in aquatic non-tetrapods (including stem gnathostomes), focusing on tissues derived from odontogenic (hypermineralized tissues, dentines and elasmodine) and osteogenic (bone tissues) cell condensations. The novelty of our new scenario of integumentary skeletal evolution resides in the demonstration that elasmodine, the main component of elasmoid scales, is odontogenic in origin. Based on available data we propose that elasmodine is a form of lamellar dentine. Given its widespread distribution in non-tetrapod lineages we further propose that elasmodine is a very ancient tissue in vertebrates and predict that it will be found in ancestral rhombic scales and cosmoid scales.
Figures






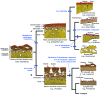
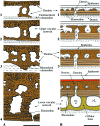
Comment in
-
'The integument story: origins, evolution and current knowledge'.J Anat. 2009 Apr;214(4):407-8. doi: 10.1111/j.1469-7580.2009.01051.x. J Anat. 2009. PMID: 19422422 Free PMC article. No abstract available.
References
-
- Arsenault M, Janvier P. The anaspid-like craniates of the Escuminac Formation (Upper Devonian) from Miguasha (Québec, Canada), with remarks on anaspid-petromyzontid relationships. In: Chang MM, Liu YH, Zhang GR, editors. Early Vertebrates and Related Problems of Evolutionary Biology. Beijing: Science Press; 1991. pp. 19–40.
-
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev. 1991;66:101–157. - PubMed
-
- Belamie E, Mosser G, Gobeaux F, Giraud-Guille MM. Possible transient liquid crystal phase during the laying out of connective tissues, a chitin and collagen as models. J Phys Condens Matter. 2006;18:115–129.
-
- Bemis WE, Northcutt RG. Skin and blood vessels of the snout of the Australian lungfish, Neoceratodus forsteri, and their significance for interpreting the cosmine of Devonian lungfishes. Acta Zool (Stockh) 1992;73:115–139.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

