The integumentary skeleton of tetrapods: origin, evolution, and development
- PMID: 19422424
- PMCID: PMC2736118
- DOI: 10.1111/j.1469-7580.2008.01043.x
The integumentary skeleton of tetrapods: origin, evolution, and development
Abstract
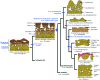
Although often overlooked, the integument of many tetrapods is reinforced by a morphologically and structurally diverse assemblage of skeletal elements. These elements are widely understood to be derivatives of the once all-encompassing dermal skeleton of stem-gnathostomes but most details of their evolution and development remain confused and uncertain. Herein we re-evaluate the tetrapod integumentary skeleton by integrating comparative developmental and tissue structure data. Three types of tetrapod integumentary elements are recognized: (1) osteoderms, common to representatives of most major taxonomic lineages; (2) dermal scales, unique to gymnophionans; and (3) the lamina calcarea, an enigmatic tissue found only in some anurans. As presently understood, all are derivatives of the ancestral cosmoid scale and all originate from scleroblastic neural crest cells. Osteoderms are plesiomorphic for tetrapods but demonstrate considerable lineage-specific variability in size, shape, and tissue structure and composition. While metaplastic ossification often plays a role in osteoderm development, it is not the exclusive mode of skeletogenesis. All osteoderms share a common origin within the dermis (at or adjacent to the stratum superficiale) and are composed primarily (but not exclusively) of osseous tissue. These data support the notion that all osteoderms are derivatives of a neural crest-derived osteogenic cell population (with possible matrix contributions from the overlying epidermis) and share a deep homology associated with the skeletogenic competence of the dermis. Gymnophionan dermal scales are structurally similar to the elasmoid scales of most teleosts and are not comparable with osteoderms. Whereas details of development are lacking, it is hypothesized that dermal scales are derivatives of an odontogenic neural crest cell population and that skeletogenesis is comparable with the formation of elasmoid scales. Little is known about the lamina calcarea. It is proposed that this tissue layer is also odontogenic in origin, but clearly further study is necessary. Although not homologous as organs, all elements of the integumentary skeleton share a basic and essential relationship with the integument, connecting them with the ancestral rhombic scale.
Figures



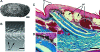

Comment in
-
'The integument story: origins, evolution and current knowledge'.J Anat. 2009 Apr;214(4):407-8. doi: 10.1111/j.1469-7580.2009.01051.x. J Anat. 2009. PMID: 19422422 Free PMC article. No abstract available.
References
-
- Ahlberg PE. A re-examination of sarcopterygian interrelationships, with special reference to the Porolepiformes. Zool J Linn Soc. 1991;103:241–287.
-
- Alexander RMN, Fariña R, Vizcaíno SF. Tail blow energy and carapace fractures in a large glyptodont (Mammalia, Xenarthra) Zool J Linn Soc. 1999;126:41–46.
-
- Anderson JS. Incorporating ontogeny into the matrix: a phylogenetic evaluation of developmental evidence for the origin of modern amphibians. In: Anderson JS, Sues H-D, editors. Major Transitions in Vertebrate Evolution. Bloomington, IN: Indiana University Press; 2007. pp. 182–227.
-
- Anderson JS, Reisz RR, Scott D, Frobisch NB, Sumida SS. A stem batrachian from the Early Permian of Texas and the origin of frogs and salamanders. Nature. 2008;453:515–518. - PubMed
-
- Barrett PM, Clarck J, Brinkman DB, Chapman SD, Ensom PC. Morphology, histology and identification of the ‘granicones’ from the Purbeck Limestone Formation (Lower Cretaceous: Berriasian) of Dorset, southern England. Cretac Res. 2002;23:279–295.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

