Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia
- PMID: 19422428
- PMCID: PMC2736122
- DOI: 10.1111/j.1469-7580.2009.01066.x
Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia
Abstract
Historically, the term 'keratin' stood for all of the proteins extracted from skin modifications, such as horns, claws and hooves. Subsequently, it was realized that this keratin is actually a mixture of keratins, keratin filament-associated proteins and other proteins, such as enzymes. Keratins were then defined as certain filament-forming proteins with specific physicochemical properties and extracted from the cornified layer of the epidermis, whereas those filament-forming proteins that were extracted from the living layers of the epidermis were grouped as 'prekeratins' or 'cytokeratins'. Currently, the term 'keratin' covers all intermediate filament-forming proteins with specific physicochemical properties and produced in any vertebrate epithelia. Similarly, the nomenclature of epithelia as cornified, keratinized or non-keratinized is based historically on the notion that only the epidermis of skin modifications such as horns, claws and hooves is cornified, that the non-modified epidermis is a keratinized stratified epithelium, and that all other stratified and non-stratified epithelia are non-keratinized epithelia. At this point in time, the concepts of keratins and of keratinized or cornified epithelia need clarification and revision concerning the structure and function of keratin and keratin filaments in various epithelia of different species, as well as of keratin genes and their modifications, in view of recent research, such as the sequencing of keratin proteins and their genes, cell culture, transfection of epithelial cells, immunohistochemistry and immunoblotting. Recently, new functions of keratins and keratin filaments in cell signaling and intracellular vesicle transport have been discovered. It is currently understood that all stratified epithelia are keratinized and that some of these keratinized stratified epithelia cornify by forming a Stratum corneum. The processes of keratinization and cornification in skin modifications are different especially with respect to the keratins that are produced. Future research in keratins will provide a better understanding of the processes of keratinization and cornification of stratified epithelia, including those of skin modifications, of the adaptability of epithelia in general, of skin diseases, and of the changes in structure and function of epithelia in the course of evolution. This review focuses on keratins and keratin filaments in mammalian tissue but keratins in the tissues of some other vertebrates are also considered.
Figures
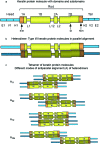
Comment in
-
'The integument story: origins, evolution and current knowledge'.J Anat. 2009 Apr;214(4):407-8. doi: 10.1111/j.1469-7580.2009.01051.x. J Anat. 2009. PMID: 19422422 Free PMC article. No abstract available.
References
-
- Achtstätter T, Moll R, Moore B, Franke WW. Cytokeratin polypeptide patterns of different epithelia of the human male urogenital tract: Immunofluorescence and gel electrophoretic studies. J Histochem Cytochem. 1985;33:415–426. - PubMed
-
- Ahvazi B, Boeshans KM, Idler W, Baxa U, Steinert PM. Roles of calcium ions in the activation and activity of the transglutaminase 3 enzyme. J Biol Chem. 2003;278:23834–23841. - PubMed
-
- Alibardi L, Toni M. Immuno-cross reactivity of transglutaminase and cornification marker proteins in the epidermis of vertebrates suggests common processes of soft cornification across species. Mol Dev Evol. 2004;302:526–549. - PubMed
-
- Alibardi L, Maurizil MG, Taddei C. Immunocytochemical and electrophoretic distribution of cytokeratins in the regenerating epidermis of the lizard podarcis muralis. J Morphol. 2000;246:179–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

