Evolution of hard proteins in the sauropsid integument in relation to the cornification of skin derivatives in amniotes
- PMID: 19422429
- PMCID: PMC2736123
- DOI: 10.1111/j.1469-7580.2009.01045.x
Evolution of hard proteins in the sauropsid integument in relation to the cornification of skin derivatives in amniotes
Abstract
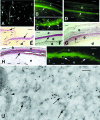
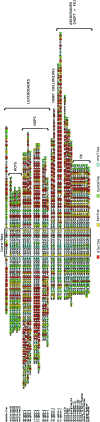
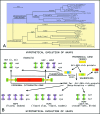
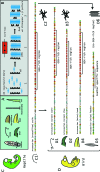
Hard skin appendages in amniotes comprise scales, feathers and hairs. The cell organization of these appendages probably derived from the localization of specialized areas of dermal-epidermal interaction in the integument. The horny scales and the other derivatives were formed from large areas of dermal-epidermal interaction. The evolution of these skin appendages was characterized by the production of specific coiled-coil keratins and associated proteins in the inter-filament matrix. Unlike mammalian keratin-associated proteins, those of sauropsids contain a double beta-folded sequence of about 20 amino acids, known as the core-box. The core-box shows 60%-95% sequence identity with known reptilian and avian proteins. The core-box determines the polymerization of these proteins into filaments indicated as beta-keratin filaments. The nucleotide and derived amino acid sequences for these sauropsid keratin-associated proteins are presented in conjunction with a hypothesis about their evolution in reptiles-birds compared to mammalian keratin-associated proteins. It is suggested that genes coding for ancestral glycine-serine-rich sequences of alpha-keratins produced a new class of small matrix proteins. In sauropsids, matrix proteins may have originated after mutation and enrichment in proline, probably in a central region of the ancestral protein. This mutation gave rise to the core-box, and other regions of the original protein evolved differently in the various reptilians orders. In lepidosaurians, two main groups, the high glycine proline and the high cysteine proline proteins, were formed. In archosaurians and chelonians two main groups later diversified into the high glycine proline tyrosine, non-feather proteins, and into the glycine-tyrosine-poor group of feather proteins, which evolved in birds. The latter proteins were particularly suited for making the elongated barb/barbule cells of feathers. In therapsids-mammals, mutations of the ancestral proteins formed the high glycine-tyrosine or the high cysteine proteins but no core-box was produced in the matrix proteins of the hard corneous material of mammalian derivatives.
Figures

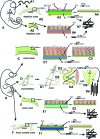
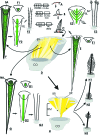
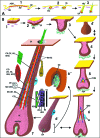
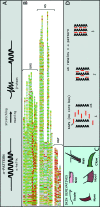






Comment in
-
'The integument story: origins, evolution and current knowledge'.J Anat. 2009 Apr;214(4):407-8. doi: 10.1111/j.1469-7580.2009.01051.x. J Anat. 2009. PMID: 19422422 Free PMC article. No abstract available.
References
-
- Alibardi L. Keratinization and lipogenesis in epidermal derivatives of the zebrafinch, Taeniopygia guttata castanotis (Aves, Passeriformes, Ploecidae) during embryonic development. J Morphol. 2002;251:294–308. - PubMed
-
- Alibardi L. Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. J Exp Zoolog B Mol Dev Evol. 2003;298:12–41. - PubMed
-
- Alibardi L. Dermo-epidermal interactions in reptilian scales: speculations on the evolution of scales, feathers, and hairs. J Exp Zoolog B Mol Dev Evol. 2004b;302:365–383. - PubMed
-
- Alibardi L. Fine structure and immunocytochemistry of monotreme hairs, with emphasis on the inner root sheath and trichohyalin-based cornification during hair evolution. J Morphol. 2004c;261:345–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

