Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function
- PMID: 19422785
- PMCID: PMC2757529
- DOI: 10.1016/j.bbamem.2009.04.020
Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function
Abstract
The ability of phospholipids to act as determinants of membrane protein structure and function is probably best exemplified by cardiolipin (CL), the signature phospholipid of mitochondria. Early efforts to reconstitute individual respiratory complexes and members of the mitochondrial carrier family, most notably the ADP/ATP carrier (AAC), often demonstrated the importance of CL. Over the past decade, the significance of CL in the organization of components of the electron transport chain into higher order assemblies, termed respiratory supercomplexes, has been established. Another protein required for oxidative phosphorylation, AAC, has received comparatively little attention likely stemming from the fact that AACs were thought to function in isolation as either homodimers or monomers. Recently however, AACs have been demonstrated to interact with the respiratory supercomplex, other members of the mitochondrial carrier family, and the TIM23 translocon. Interestingly, many if not all of these interactions depend on CL. As the paradigm for the mitochondrial carrier family, these discoveries with AAC suggest that other members of this large group of important proteins may be more gregarious than anticipated. Moreover, it is proposed that AAC and perhaps additional members of the mitochondrial carrier family might represent downstream targets of pathological states involving alterations in CL.
Figures

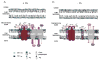
References
-
- Hunte C. Specific protein-lipid interactions in membrane proteins. Biochem Soc Trans. 2005;33:938–942. - PubMed
-
- Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem. 2000;275:22387–22394. - PubMed
-
- Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

