Human Freud-2/CC2D1B: a novel repressor of postsynaptic serotonin-1A receptor expression
- PMID: 19423080
- PMCID: PMC4084727
- DOI: 10.1016/j.biopsych.2009.02.033
Human Freud-2/CC2D1B: a novel repressor of postsynaptic serotonin-1A receptor expression
Abstract
Background: Altered expression of serotonin-1A (5-HT1A) receptors, both presynaptic in the raphe nuclei and post-synaptic in limbic and cortical target areas, has been implicated in mood disorders such as major depression and anxiety. Within the 5-HT1A receptor gene, a powerful dual repressor element (DRE) is regulated by two protein complexes: Freud-1/CC2D1A and a second, unknown repressor. Here we identify human Freud-2/CC2D1B, a Freud-1 homologue, as the second repressor.
Methods: Freud-2 distribution was examined with Northern and Western blot, reverse transcriptase polymerase chain reaction, and immunohistochemistry/immunofluorescence; Freud-2 function was examined by electrophoretic mobility shift, reporter assay, and Western blot.
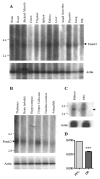
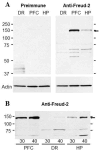
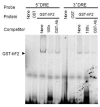
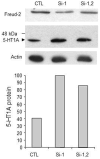
Results: Freud-2 RNA was widely distributed in brain and peripheral tissues. Freud-2 protein was enriched in the nuclear fraction of human prefrontal cortex and hippocampus but was weakly expressed in the dorsal raphe nucleus. Freud-2 immunostaining was co-localized with 5-HT1A receptors, neuronal and glial markers. In prefrontal cortex, Freud-2 was expressed at similar levels in control and depressed male subjects. Recombinant hFreud-2 protein bound specifically to 5' or 3' human DRE adjacent to the Freud-1 site. Human Freud-2 showed strong repressor activity at the human 5-HT1A or heterologous promoter in human HEK-293 5-HT1A-negative cells and neuronal SK-N-SH cells, a model of postsynaptic 5-HT1A receptor-positive cells. Furthermore, small interfering RNA knockdown of endogenous hFreud-2 expression de-repressed 5-HT1A promoter activity and increased levels of 5-HT1A receptor protein in SK-N-SH cells.
Conclusions: Human Freud-2 binds to the 5-HT1A DRE and represses the human 5-HT1A receptor gene to regulate its expression in non-serotonergic cells and neurons.
Figures





Similar articles
-
Freud-2/CC2D1B mediates dual repression of the serotonin-1A receptor gene.Eur J Neurosci. 2011 Jan;33(2):214-23. doi: 10.1111/j.1460-9568.2010.07498.x. Epub 2010 Dec 14. Eur J Neurosci. 2011. PMID: 21155902 Free PMC article.
-
Cell type-dependent recruitment of trichostatin A-sensitive repression of the human 5-HT1A receptor gene.J Neurochem. 2004 Feb;88(4):857-68. doi: 10.1046/j.1471-4159.2003.02223.x. J Neurochem. 2004. PMID: 14756806
-
Freud-1: A neuronal calcium-regulated repressor of the 5-HT1A receptor gene.J Neurosci. 2003 Aug 13;23(19):7415-25. doi: 10.1523/JNEUROSCI.23-19-07415.2003. J Neurosci. 2003. PMID: 12917378 Free PMC article.
-
Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness.Mol Brain. 2011 May 27;4:21. doi: 10.1186/1756-6606-4-21. Mol Brain. 2011. PMID: 21619616 Free PMC article. Review.
-
Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression.Front Behav Neurosci. 2014 Jun 6;8:199. doi: 10.3389/fnbeh.2014.00199. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24936175 Free PMC article. Review.
Cited by
-
hsa-miR-3177-5p and hsa-miR-3178 Inhibit 5-HT1A Expression by Binding the 3'-UTR Region in vitro.Front Mol Neurosci. 2019 Jan 31;12:13. doi: 10.3389/fnmol.2019.00013. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30766477 Free PMC article.
-
LRRK2 G2019S Induces Anxiety/Depression-like Behavior before the Onset of Motor Dysfunction with 5-HT1A Receptor Upregulation in Mice.J Neurosci. 2018 Feb 14;38(7):1611-1621. doi: 10.1523/JNEUROSCI.4051-15.2017. Epub 2018 Jan 5. J Neurosci. 2018. PMID: 29305532 Free PMC article.
-
The Mammalian Orthologs of Drosophila Lgd, CC2D1A and CC2D1B, Function in the Endocytic Pathway, but Their Individual Loss of Function Does Not Affect Notch Signalling.PLoS Genet. 2015 Dec 31;11(12):e1005749. doi: 10.1371/journal.pgen.1005749. eCollection 2015 Dec. PLoS Genet. 2015. PMID: 26720614 Free PMC article.
-
Abrogated Freud-1/Cc2d1a Repression of 5-HT1A Autoreceptors Induces Fluoxetine-Resistant Anxiety/Depression-Like Behavior.J Neurosci. 2017 Dec 6;37(49):11967-11978. doi: 10.1523/JNEUROSCI.1668-17.2017. Epub 2017 Nov 3. J Neurosci. 2017. PMID: 29101244 Free PMC article.
-
Recruitment by the Repressor Freud-1 of Histone Deacetylase-Brg1 Chromatin Remodeling Complexes to Strengthen HTR1A Gene Repression.Mol Neurobiol. 2017 Dec;54(10):8263-8277. doi: 10.1007/s12035-016-0306-4. Epub 2016 Dec 2. Mol Neurobiol. 2017. PMID: 27914010 Free PMC article.
References
-
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. Journal of Biological Chemistry. 1990;265:5825–5832. - PubMed
-
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. - PubMed
-
- Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58:947–954. - PubMed
-
- Pitchot W, Hansenne M, Pinto E, Reggers J, Fuchs S, Ansseau M. 5-Hydroxytryptamine 1A receptors, major depression, and suicidal behavior. Biol Psychiatry. 2005;58:854–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

