Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties
- PMID: 19423507
- PMCID: PMC2726858
- DOI: 10.1242/jeb.028621
Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties
Abstract
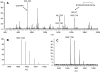
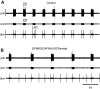
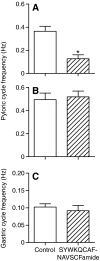
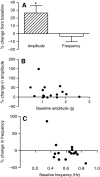
The allatostatins comprise three structurally distinct peptide families that regulate juvenile hormone production by the insect corpora allata. A-type family members contain the C-terminal motif -YXFGLamide and have been found in species from numerous arthropod taxa. Members of the B-type family exhibit a -WX(6)Wamide C-terminus and, like the A-type peptides, appear to be broadly conserved within the Arthropoda. By contrast, members of the C-type family, typified by the unblocked C-terminus -PISCF, a pyroglutamine blocked N-terminus, and a disulfide bridge between two internal Cys residues, have only been found in holometabolous insects, i.e. lepidopterans and dipterans. Here, using transcriptomics, we have identified SYWKQCAFNAVSCFamide (disulfide bridging predicted between the two Cys residues), a known honeybee and water flea C-type-like peptide, from the American lobster Homarus americanus (infraorder Astacidea). Using matrix assisted laser desorption/ionization Fourier transform mass spectrometry (MALDI-FTMS), a mass corresponding to that of SYWKQCAFNAVSCFamide was detected in the H. americanus brain, supporting the existence of this peptide and its theorized structure. Furthermore, SYWKQCAFNAVSCFamide was detected by MALDI-FTMS in neural tissues from five additional astacideans as well as 19 members of four other decapod infraorders (i.e. Achelata, Anomura, Brachyura and Thalassinidea), suggesting that it is a broadly conserved decapod peptide. In H. americanus, SYWKQCAFNAVSCFamide is capable of modulating the output of both the pyloric circuit of the stomatogastric nervous system and the heart. This is the first demonstration of bioactivity for this peptide in any species.
Figures



References
-
- Abdel-Latief, M., Meyering-Vos, M. and Hoffmann, K. H. (2004). Type-A allatostatins from the fall armyworm, Spodoptera frugiperda: molecular cloning, expression and tissue-specific localization. Arch. Insect Biochem. Physiol. 56, 120-132. - PubMed
-
- Bendtsen, J. D., Nielsen, H., von Heijne, G. and Brunak, S. (2004). Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783-795. - PubMed
-
- Birmingham, J. T., Billimoria, C. P., DeKlotz, T. R., Stewart, R. A. and Marder, E. (2003). Differential and history-dependent modulation of a stretch receptor in the stomatogastric system of the crab, Cancer borealis. J. Neurophysiol. 90, 3608-3616. - PubMed
-
- Bowser, P. R. and Tobe, S. S. (2007). Comparative genomic analysis of allatostatin-encoding (Ast) genes in Drosophila species and prediction of regulatory elements by phylogenetic footprinting. Peptides 28, 83-93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

