A protein interaction network for the large conductance Ca(2+)-activated K(+) channel in the mouse cochlea
- PMID: 19423573
- PMCID: PMC2722780
- DOI: 10.1074/mcp.M800495-MCP200
A protein interaction network for the large conductance Ca(2+)-activated K(+) channel in the mouse cochlea
Abstract
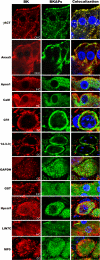
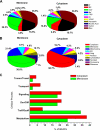
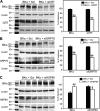
The large conductance Ca(2+)-activated K(+) or BK channel has a role in sensory/neuronal excitation, intracellular signaling, and metabolism. In the non-mammalian cochlea, the onset of BK during development correlates with increased hearing sensitivity and underlies frequency tuning in non-mammals, whereas its role is less clear in mammalian hearing. To gain insights into BK function in mammals, coimmunoprecipitation and two-dimensional PAGE, combined with mass spectrometry, were used to reveal 174 putative BKAPs from cytoplasmic and membrane/cytoskeletal fractions of mouse cochlea. Eleven BKAPs were verified using reciprocal coimmunoprecipitation, including annexin, apolipoprotein, calmodulin, hippocalcin, and myelin P0, among others. These proteins were immunocolocalized with BK in sensory and neuronal cells. A bioinformatics approach was used to mine databases to reveal binary partners and the resultant protein network, as well as to determine previous ion channel affiliations, subcellular localization, and cellular processes. The search for binary partners using the IntAct molecular interaction database produced a putative global network of 160 nodes connected with 188 edges that contained 12 major hubs. Additional mining of databases revealed that more than 50% of primary BKAPs had prior affiliations with K(+) and Ca(2+) channels. Although a majority of BKAPs are found in either the cytoplasm or membrane and contribute to cellular processes that primarily involve metabolism (30.5%) and trafficking/scaffolding (23.6%), at least 20% are mitochondrial-related. Among the BKAPs are chaperonins such as calreticulin, GRP78, and HSP60 that, when reduced with siRNAs, alter BKalpha expression in CHO cells. Studies of BKalpha in mitochondria revealed compartmentalization in sensory cells, whereas heterologous expression of a BK-DEC splice variant cloned from cochlea revealed a BK mitochondrial candidate. The studies described herein provide insights into BK-related functions that include not only cell excitation, but also cell signaling and apoptosis, and involve proteins concerned with Ca(2+) regulation, structure, and hearing loss.
Figures

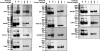

Similar articles
-
Conserved BK channel-protein interactions reveal signals relevant to cell death and survival.PLoS One. 2011;6(12):e28532. doi: 10.1371/journal.pone.0028532. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174833 Free PMC article.
-
Intrinsic disorder in the BK channel and its interactome.PLoS One. 2014 Apr 11;9(4):e94331. doi: 10.1371/journal.pone.0094331. eCollection 2014. PLoS One. 2014. PMID: 24727949 Free PMC article.
-
Expression of BK-type calcium-activated potassium channel splice variants during chick cochlear development.J Comp Neurol. 2010 Jul 1;518(13):2554-69. doi: 10.1002/cne.22352. J Comp Neurol. 2010. PMID: 20503427 Free PMC article.
-
Protein Network Interacting with BK Channels.Int Rev Neurobiol. 2016;128:127-61. doi: 10.1016/bs.irn.2016.03.003. Epub 2016 Mar 28. Int Rev Neurobiol. 2016. PMID: 27238263 Review.
-
Functional Role of Mitochondrial and Nuclear BK Channels.Int Rev Neurobiol. 2016;128:163-91. doi: 10.1016/bs.irn.2016.03.018. Epub 2016 Apr 19. Int Rev Neurobiol. 2016. PMID: 27238264 Review.
Cited by
-
The mitochondrial BKCa channel cardiac interactome reveals BKCa association with the mitochondrial import receptor subunit Tom22, and the adenine nucleotide translocator.Mitochondrion. 2017 Mar;33:84-101. doi: 10.1016/j.mito.2016.08.017. Epub 2016 Aug 31. Mitochondrion. 2017. PMID: 27592226 Free PMC article.
-
Current Challenges of Mitochondrial Potassium Channel Research.Front Physiol. 2022 May 31;13:907015. doi: 10.3389/fphys.2022.907015. eCollection 2022. Front Physiol. 2022. PMID: 35711307 Free PMC article. Review.
-
BKCa channel regulates calcium oscillations induced by alpha-2-macroglobulin in human myometrial smooth muscle cells.Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):E2335-44. doi: 10.1073/pnas.1516863113. Epub 2016 Apr 4. Proc Natl Acad Sci U S A. 2016. PMID: 27044074 Free PMC article.
-
Intracellular BK(Ca) (iBK(Ca)) channels.J Physiol. 2012 Dec 1;590(23):5937-47. doi: 10.1113/jphysiol.2011.215533. Epub 2012 Aug 28. J Physiol. 2012. PMID: 22930268 Free PMC article. Review.
-
The large conductance calcium-activated potassium channel affects extrinsic and intrinsic mechanisms of apoptosis.J Neurosci Res. 2015 May;93(5):745-54. doi: 10.1002/jnr.23538. Epub 2015 Jan 7. J Neurosci Res. 2015. PMID: 25581503 Free PMC article.
References
-
- Marty A. ( 1981) Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 291, 497– 500 - PubMed
-
- Brainard A. M., Miller A. J., Martens J. R., England S. K. ( 2005) Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am. J. Physiol. Cell Physiol. 289, C49– C57 - PubMed
-
- Robitaille R., Adler E. M., Charlton M. P. ( 1993) Calcium channels and calcium-gated potassium channels at the frog neuromuscular junction. J. Physiol. Paris 87, 15– 24 - PubMed
-
- Pattillo J. M., Yazejian B., DiGregorio D. A., Vergara J. L., Grinnell A. D., Meriney S. D. ( 2001) Contribution of presynaptic calcium-activated potassium currents to transmitter release regulation in cultured Xenopus nerve-muscle synapses. Neuroscience 102, 229– 240 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

